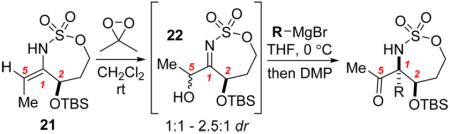
Table 1.
| entry | R | product | yield | dr a |
|---|---|---|---|---|
| 1b | Et | 16 | 29%c | 1.9:1 |
| 2 | CH=CH2 | 23 | 45% | 2:1 |
| 3 | C≡CH | 24 | 48% | 2.3:1 |
| 4d | C≡CH | 24 | 68% | 11.5:1 |
Based on 1H NMR analysis of crude product.
Reaction using CH2Cl2 as the solvent.
Isolated yield and dr are before DMP oxidation. Hydride reduction was a significant byproduct observed in 18% yield.
Grignard prestirred at 0 °C for 1 h prior to addition. Yield refers to isolated material.