Abstract
Extreme heat has been associated with increased mortality, particularly in temperate climates. Few epidemiologic studies have considered the Pacific Northwest region in their analyses. This study quantified the historical (May to September, 1980–2010) heat-mortality relationship in the most populous Pacific Northwest County, King County, Washington. A relative risk (RR) analysis was used to explore the relationship between heat and all-cause mortality on 99th percentile heat days, while a time series analysis, using a piece-wise linear model fit, was used to estimate the effect of heat intensity on mortality, adjusted for temporal trends. For all ages, all causes, we found a 10 % (1.10 (95 % confidence interval (CI), 1.06, 1.14)) increase in the risk of death on a heat day versus non-heat day. When considering the intensity effect of heat on all-cause mortality, we found a 1.69 % (95 % CI, 0.69, 2.70) increase in the risk of death per unit of humidex above 36.0 °C. Mortality stratified by cause and age produced statistically significant results using both types of analyses for: all-cause, non-traumatic, circulatory, cardiovascular, cerebrovascular, and diabetes causes of death. All-cause mortality was statistically significantly modified by the type of synoptic weather type. These results demonstrate that heat, expressed as humidex, is associated with increased mortality on heat days, and that risk increases with heat’s intensity. While age was the only individual-level characteristic found to modify mortality risks, statistically significant increases in diabetes-related mortality for the 45–64 age group suggests that underlying health status may contribute to these risks.
Keywords: Climate change, Extreme heat, Mortality, Washington State
Introduction
Extreme-heat events have contributed to thousands of deaths in the USA, Canada, and Europe since the early 1980s (Anderson and Bell 2009, 2011; Jackson et al. 2010; Baccini et al. 2008; Basu et al. 2008; Naughton 2002; Whitman et al. 1997). V-, U-, or J-shaped relationships have been identified where there is a minimum mortality temperature (also called “threshold” or “turning point) beyond which mortality increases significantly with increasing heat (Baccini et al. 2008; Kim et al. 2006; Curriero et al. 2002). Studies have also suggested that the heat-mortality relationship may not be the same in all locations (Baccini et al. 2008; Curriero et al. 2002). Curriero et al. 2002 research demonstrated a stronger association in mortality risk for northern cities in the USA, at lower temperatures, when compared with southern cities.
Other factors have been identified as modifying heat’s influence on mortality rates including intensity and duration of the described heat event (Anderson and Bell 2011; Baccini et al. 2008), synoptic weather patterns (Kalkstein et al. 2011; Sheridan et al. 2009), access to air conditioning (Naughton 2002; O’Neill et al. 2005), social isolation (Naughton 2002; Kaiser et al. 2001), socio-economic status (Jones et al. 1982; Kaiser et al. 2001), and ethnicity and educational status (O’Neill et al. 2003). It is important to note that across all of the above-referenced studies, there is significant variability in the results—suggesting that place or regionality matters. Yardley et al. (2011) suggest that the spatial distribution in heat-related mortality indicates more at play than just temperature and physiology and that the addition of neighborhood or area-level characteristics would further explain local heat-risk differences. For example, Harlan et al. (2006) found that microclimates, or urban heat islands, affect mortality risk and that lower socio-economic status groups were more likely to occupy these areas. Davis et al. (2004) found that communities concentrating on planning improvements to increase shade and access to water decreased their heat-related mortality. Finally, Stone et al. (2010) found that metropolitan sprawl affects the rate of increase in annual number of extreme-heat events.
In addition to community infrastructure, it is also believed that the social connectedness of place affects heat-related health risks. Smoyer (1998), Klinenberg (2002), and Naughton (2002) have all found that social connections, perception of safety, and the community’s walkability affect heat mortality. As average temperatures and the frequency of extreme-heat events are predicted to increase with climate change, understanding the regional heat-mortality relationship becomes increasingly important to direct adaptation-related policy decisions.
This study investigated the relationship between heat and mortality in the most populous Pacific Northwest County, King County, Washington (King County 2012). Two different modeling approaches were used. We first built upon our previous work that found significant increases in non-traumatic mortality associated with heat days compared with non-heat days in the Pacific Northwest region (Jackson et al. 2010). We used a relative risk (RR) model to explore an expanded list of age-adjusted, cause-of-death categories requested by the local health jurisdiction. Second, we added a time series analysis to study heat intensity effects on mortality. Specifically, we quantified the percentage increase in mortality associated with a 1° increase in humidex for the same expanded list of age-adjusted, cause-of-death categories. Using the time series model, we were able to explore effect modification from individual-level characteristics, as well as analyze other effects from heat, including cool-down, duration, and lag effects. To our knowledge, no other study has looked at such a comprehensive list of mortality categories in the Pacific Northwest, using two methods of analyses.
Materials and methods
Mortality and population data
King County death certificate data for all causes, 1980 to 2010, were obtained from the Washington State Department of Health. Only deaths which occurred during the summer months of May through September were analyzed. There are 153 days/constrained calendar year, a total of 4743 days for the entire study period. Death certificates were coded using the International Classification of Diseases (ICD). ICD-9 codes were used from 1980 to 1998 and ICD-10 codes from 1999 to 2010.
This study expanded the cause-of-death categories analyzed by Jackson et al. (2010) and Isaksen et al. (2014) from only non-traumatic causes (ICD-9 001-799, ICD-10 A01-R99) to all-cause mortality (ICD-9 000, ICD-10 A00+). We also investigated select subsets of all-cause mortality that were determined a priori through the literature (Jackson et al. 2010; Isaksen et al. 2014; Cheng et al. 2005) or that were specifically requested by the local health jurisdiction. These subsets include: diabetes, circulatory, cardiovascular, ischemic, cerebrovascular, respiratory, nephritis and nephrotic, acute renal failure, mental disorders, and natural heat exposure including dehydration. Our analyses also made use of the death certificate database variable, Inj_cause, which describes whether a death is classified as natural, accidental, suicide, or homicide. Table 1 lists the specific ICD-9 and ICD-10 codes used in this study.
Table 1.
Underlying causes of death and associated international classification of disease (ICD)-9 (1980 to 1998) and ICD-10 (1999–2010) codes
| Category | ICD-9 code | ICD-10 code |
|---|---|---|
| All causes | 000+ | A00+ |
| Non-traumatic | 001–799 | A00–R99 |
| Select non-traumatic causes | ||
| Diabetes | 250 | E08–E13 |
| Circulatory | 390–459 | I00–I99, G45, G46 |
| Cardiovascular | 393–429 | I05–I52 |
| Ischemic | 410–414 | I20–I25 |
| Cerebrovascular | 430–438 | I67 |
| Respiratory | 460–519 | J00–J99 |
| Nephritis and nephrotic syndromes | 580–589 | N17–N19 |
| Acute renal failure | 584 | N17 |
| Mental disorders | 290–316 | F01–F69 |
| Select traumatic causes | ||
| Natural heat exposure and dehydrationa | E900.0 or E900.9 and 992 | X30 and T67 |
| Accidentb | 2 | 2 |
| Suicideb | 3 | 3 |
| Homicideb | 4 | 4 |
While mortality attributed to natural heat-related exposure is classified as a traumatic external injury, we have included the non-traumatic dehydration code ICD-9 276.51/ICD-10 E86.0 with this subgrouping. We included dehydration in this group to ensure we captured cases potentially misclassified, given the limited use and possible recognition of existing heat-related exposure ICD-9 and ICD-10 codes
The Washington State Department of Health codes the variable “Inj_caus” as the type of external injury indicated by the underlying cause of death: accident=2; suicide=3; and homicide=4
A priori, we anticipated that several individual-level characteristics might identify populations that are vulnerable to heat-related mortality: age, non-White race, less than a high school education, Hispanic origin, and tobacco contribution to death. Population data, by age groups (0–4, 5–14, 15–44, 45–64, 65–84, 85+), were obtained from the Washington State Office of Financial Management (OFM) (Washington State Office of Financial Management 2012).
Meteorology data
This study used a historical 1/16° resolution gridded meteorological data set produced by the University of Washington’s Climate Impacts Group (Maurer et al. 2002). The climatologic foundation of this data set is the Parameter-Elevation Relationships on Independent Slopes Model (PRISM), developed by Oregon State University (PRISM Climate Group). PRISM is considered to represent the most current knowledge on spatial climatic patterns for the USA (Daly et al. 2008). Daily temperature and relative humidity values were constructed using PRISM’s regional spatial climatic patterns and weather station observations from the Global Historical Climate Network-Daily (GHCN). GHCN is a National Oceanic and Atmospheric Administration database of daily meteorological measurements from land surface stations across the globe (NOAA Satellite and Information Service 2009). The resulting meteorology data set used for this study contains daily maximum/minimum temperature, precipitation, and relative humidity values for each meteorological center point. The county-wide daily maximum temperature and average relative humidity values were used to construct our exposure metric, humidex.
Exposure assessment
As with our previous studies (Jackson et al. 2010; Isaksen et al. 2014), humidex was used as the measure of exposure. While there are other measures of heat (e.g., mean, minimum, maximum temperature, as well as apparent temperature) that can be used to model heat-mortality relationships, we chose to use humidex in order to facilitate comparison across previous studies. Furthermore, Barnett et al. (2010) reviewed multiple measures of temperature (including humidex) to determine the best predictor of heat mortality. While they found no measure was consistently superior to others, humidex was found to perform as well, or better, over multiple age groups, seasons, and regions (Barnett et al. 2010).
Humidex is a feels-like index that measures the combined effects of temperature and humidity on the human body (Masterton and Richardson 1979). For this study, an average daily maximum humidex was computed over all meteorological center points located in King County. Exposure to heat was then estimated as the county-wide average maximum humidex value for each day within the study’s time frame. Humidex is defined by the following formula and is expressed in units of degrees Celsius:
| (1) |
where T is the air temperature (°C), H is the average relative humidity (%), and v is the vapor pressure (kPa) (Canadian Centre for Occupational Health and Safety 2011). In this analysis, “humidex” refers to the “county-wide average daily maximum humidex.”
Association between humidex and mortality
Relative risk analysis
A heat day was defined as a day in which the humidex exceeded a specified threshold. Jackson et al. (2010) used the 99th percentile of the average greater Seattle area-wide (King, Pierce, and Snohomish counties) maximum humidex as the threshold to define a heat day. In this analysis, we tried the 99th, 95th, and 90th percentiles for King County and chose the one that gave the best fit to the data (maximum likelihood). The following Poisson regression model was used:
| (2) |
where Yjis the mortality count on day j, Pj is the population, λj is the mortality incidence rate of a non-heat day, {humidexj >threshold} is the indicator of a heat day, and β1I is the change in mortality rate on a heat day. This approach modeled the expected mortality count after controlling for population growth.
Time series analysis
A Poisson model was built to explore the relationship between daily humidex and mortality rates. Similar to other studies (Anderson and Bell 2009; Baccini et al. 2008; Curriero et al. 2002), we used non-parametric splines to model the log-mortality rate over time and humidex. Specifically, we assumed that:
| (3) |
where μj is the expected mortality rate on day j, s(hj) is a penalized regression spline modeling the effects of humidex, s(tj) is a penalized regression spline modeling the temporal trend of mortality over 31 years, and (βl’s) is the adjustment for seasonal monthly effects.
To increase interpretability and usefulness for public health practitioners and policymakers, we simplified the non-parametric spline model with a piece-wise linear model, fit with two knots. The first knot was set at the 50th percentile of summer-time humidex values. The second knot, or “optimal alert threshold” for humidex, was identified by exploring 0.° incremental changes starting at 20 °C and continuing through 44 °C humidex to maximize the likelihood of the following model:
| (4) |
where hjis the humidex value on day j, hq50 is the 50th percentile of humidex from May to September, 1980–2010, ĥ0 is the optimal alert threshold, s(tj) is a natural cubic spline modeling the temporal trend of mortality over 31 years, and Imonth is the indicator variable for months May through September. A heat day was then defined as a day in which the humidex exceeded the optimal alert threshold. The impact of heat intensity on mortality was assessed by the slope of the line above the threshold. The “mgcv” and “GAM” packages were used with the statistical software R version 2.14.1 to determine the model’s degrees of freedom for the temporal trend and to tune the threshold, respectively (R Core Team 2012).
Effect modification by individual-level characteristics
Individual-level characteristic data obtained from death certificates were evaluated for differences in mortality risk. These covariates included age, gender, race, high school graduation, marital status, Hispanic origin, and tobacco use. Effect modification was examined by adding each covariate into the model along with an interaction term. Mortality counts for all covariate groups, except age, were not adjusted by population size of the covariate group, because the data were not available. An example of exploring effect modification is illustrated by the following example of the covariate “Hispanic origin”:
| (5) |
where μijis the expected mortality rate for subpopulation with covariate level i on day j. In this example, the covariate “Hispanic origin” has two levels “Hispanic” and “non-Hispanic”. The parameter of interest is β3, the coefficient of the interaction between heat intensity indicator and the covariate. By testing the significance of the interaction between demographic variable and the heat variable, we can identify whether specific subpopulations are more vulnerable to heat intensity effects.
Other heat effects on mortality
Several studies have suggested that cooler night-time temperatures help minimize the effect of heat on mortality (Schwartz 2005), where lengthier heat events increase mortality risk (D’Ippoliti et al. 2010; Anderson and Bell 2011), and that the type of synoptic weather pattern may influence mortality rates on heat days (Kalkstein and Greene 1997; Sheridan et al. 2009). This study evaluated the data to see if there was a “cool-down effect,” whereas an elevated minimum humidex on a hot day contributed to an increase in mortality beyond the effect of the maximum humidex during the day (Eq. 6). The study also evaluated whether the magnitude of humidex above the optimal alert threshold affected risk (Eq. 7). For data on heat days, cool-down effect is explored using the following two models:
| (6) |
| (7) |
where difference is defined as the daily maximum humidex—daily minimum humidex for a given heat day, and aboveThres is defined as the daily maximum humidex—threshold.
Similarly, this study examined the relationship between mortality count and heat event duration (number of consecutive heat days). For data on heat days, a duration effect is explored using the following two models:
| (8) |
| (9) |
where duration is defined as the day’s order in a given heat event.
This study also explored whether or not there was a lag effect between humidex and mortality over several days. A lag effect can best be described as the total heat effect on mortality spread over several days or weeks. Following the methods described in Armstrong (2006), we explored distributed lag effects using the following model:
| (10) |
where Yj is the mortality count on day j, s(time) is a spline curve over time, and f(h~j) is a function of historic humidex values on days up to day j, which takes a weighted effect of humidex on day j and the previous L days. By assuming different constraints or temporal structures for βl, l=0,…,L, we examined the evidence of lag effects. This was implemented using the “dlnm” (distributed lag non-linear model) package in R version 2.14.1 (Gasparrini 2011).
Finally, we investigated whether or not mortality rates were influenced by the type of synoptic weather (ambient weather conditions) on a given heat day. Previous research indicates that moist and dry tropical air masses are associated with increased mortality (Kalkstein et al. 2011; Sheridan et al. 2009). Using daily spatial synoptic classification data for the Seattle/Tacoma station (Sheridan 2013), effect modification was explored by adding synoptic classification as a covariate into the model along with an interaction term, Eq. (5).
Results
King County accounted for approximately 30 % of Washington State’s population throughout the study’s time frame (Washington State Office of Financial Management 2012). From 1980 to 2010, the county’s population increased over 52 %, with age groups 45–64, 65–84, and 85+ increasing 110, 52.6, and 150 %, respectively (Table 2). King County is located in Western Washington and is characterized by relatively cool summers. Its summer humidex values range from a minimum average of 6.66 °C (44.0 °F) to a maximum average of 22.4 °C (72.3 °F) (Table 3). From 1980 to 2010 (May–September), King County experienced 135,333 deaths, 34.2 % of the overall death count. On average, there were 28.5 deaths/day (Table 2). Over the study’s time frame, mortality rates remained essentially the same at 2.9/1,000 residents. Tables 2 and 3 provide descriptive demographic data, by age and percent change, and meteorological ranges, 1980–2010, respectively.
Table 2.
King County, Washington population demographics, 1980–2010
| Census population
| ||
|---|---|---|
| Census complete count | 1980 populationa | 2010 populationa |
| State total | 4,132,156 | 6,724,540 (62.7 %) |
| King County total | 1,269,749 | 1,931,249 (52.1 %) |
| % of State population | 30.7 % | 28.7 % (−7.0 %) |
| 0–4 | 78,525 | 120,294 (53.2 %) |
| 5–14 | 170,657 | 224,084 (31.3 %) |
| 15–44 | 643,707 | 856,843 (33.1 %) |
| 45–64 | 247,446 | 519,349 (110 %) |
| 65–84 | 115,910 | 176,895 (52.6 %) |
| 85+ | 13,504 | 33,784 (150 %) |
| Mortality data | ||
| 1980–2010 | All months | May–Sept. (% of total) |
| Total deaths | 395,138 | 135,333 (34.2 %) |
| Average daily mortality | 34.9 deaths/day | 28.5 deaths/day |
| Individual-level characteristics (May–Sept.) | n deaths (% of total) | |
| Gender | Male (68,203 (50.4 %)) | Female (67,130 (49.6 %)) |
| Marital status | Married or w/partner (52,098 (38.5 %)) | Other (83,235 (61.5 %)) |
| High school graduate status | ||
| High school diploma or better | 79,298 (58.6 %) | |
| No high school diploma | 53,217 (39.3 %) | |
| Status unknown | 2,818 (2.1 %) | |
| Hispanic ethnicity | ||
| Hispanic | 1,557 (1.2 %) | |
| Non-Hispanic | 133,505 (98.6 %) | |
| Unknown | 271 (0.2 %) | |
| Smoking status | ||
| Smoker | 27,287 (20.2 %) | |
| Non-smoker | 94,774 (70.0 %) | |
| Unknown | 13,272 (9.8 %) | |
| Race | ||
| White | 118,987 (87.9 %) | |
| Black | 7,027 (5.2 %) | |
| Asian/Pacific Islander | 6,935 (5.1 %) | |
| Native American | 1,184 (0.87 %) | |
| Other | 1,031 (0.76 %) | |
| Unknown | 169 (0.17 %) | |
Source: Washington State Department of Health mortality data set
Table 3.
King County, Washington meteorological descriptive data, 1980–2010
| Meteorological data (1980–2010, May–Sept.) | |
| County-wide humidex range (across all years; °C (°F)) | |
| Minimum | 6.66 °C (44.0 °F) |
| Maximum | 22.4 °C (72.3 °F) |
| Heat days above relative risk threshold (n days (% of total days)) | |
| 99th percentile 36.1 °C (97.0 °F) | 114 (2.40 %) |
| County-wide maximum humidex range | |
| Average (°C (°F)) | |
| Humidex | 38.7 °C (101.7 °F) |
| Temperature | 30.9 °C (87.6 °F) |
| Minimum (°C (°F)) | |
| Humidex | 36.1 °C (97 °F) |
| Temperature | 28.0 °C (82.4 °F) |
| Maximum (°C (°F)) | |
| Humidex | 46.3 °C (115.3 °F) |
| Temperature | 34.9 °C (94.8 °F) |
| Heat days above time series threshold (n days (% of total days)) | |
| 36.0 °C (96.8 °F) | 117 (2.47 %) |
| County-wide maximum humidex range | |
| Average (°C (°F)) | |
| Humidex | 38.6 °C (101.4 °F) |
| Temperature | 30.8 °C (87.4 °F) |
| Minimum (°C (°F)) | |
| Humidex | 36.0 °C (96.8 °F) |
| Temperature | 28.0 °C (82.4 °F) |
| Maximum (°C (°F)) | |
| Humidex | 46.3 °C (115.3 °F) |
| Temperature | 34.9 °C (94.8 °F) |
| Heat day duration | |
| Relative risk threshold 36.1 °C (97.0 °F) | Time series threshold 36.0 °C (96.8 °F) |
| Heat duration (No. of days) | No. of events | Cumulative % | Heat duration (No. of days) | No. of events | Cumulative % |
|---|---|---|---|---|---|
| 1 | 51 | 44.7 % | 1 | 52 | 44.4 % |
| 2 | 35 | 75.4 % | 2 | 35 | 74.4 % |
| 3 | 15 | 88.6 % | 3 | 17 | 88.9 % |
| 4 | 7 | 94.7 % | 4 | 7 | 94.9 % |
| 5 | 3 | 97.4 % | 5 | 3 | 97.4 % |
| 6 | 2 | 99.1 % | 6 | 2 | 99.1 % |
| 7 | 1 | 100.0 % | 7 | 1 | 100.0 % |
Association between humidex and mortality
Relative risk analysis
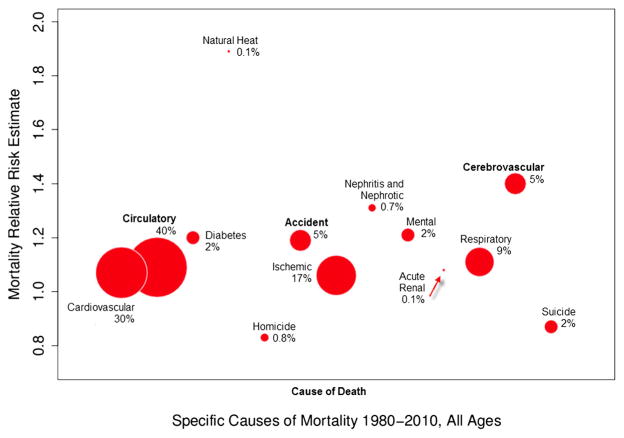
As defined by Eq. (2), a heat day for the relative risk analysis is a day that exceeds the 99th percentile (36.1 °C (97.0 °F) humidex). During 1980–2010, King County experienced 114 days over the 99th percentile. The average humidex on heat days was 38.7 °C (101.7 °F) (Table 2). For all-age, all-cause mortality, we found that the relative risk of death was 10 % greater on a heat day compared with a non-heat day. Statistically significant all-age results were found for non-traumatic (10 %), circulatory (9 %), cerebrovascular (40 %), and accident (19 %) (Table 4). To achieve a better understanding of the relative burden of death, the proportional mortality is illustrated in Fig. 1. Cause-of-death categories run along the x-axis, while the corresponding relative risk estimates are reflected by the y-axis. Figure 1 illustrates the proportion of death for each cause, in relation to the all-cause mortality on heat days (e.g., circulatory cause-of-death account for approximately 40 % of all deaths on a heat day).
Table 4.
Relative risk analysis results: increased risk (95 % CI) in mortality on a 99th percentile (36.1 °C) heat day compared with a non-heat day, by age group and cause of death
| All ages | 0–4 | 5–14 | 15–44 | 45–64 | 65–84 | 85+ | |
|---|---|---|---|---|---|---|---|
| All causes | 1.1 (1.06, 1.14) | 0.73 (0.51, 1.03) | 1.11 (0.61, 2.04) | 1.02 (0.9, 1.17) | 1.03 (0.94, 1.13) | 1.06 (1.01, 1.12) | 1.18 (1.11, 1.26) |
| Non-traumatic | 1.1 (1.06, 1.14) | 0.75 (0.53, 1.08) | 1.07 (0.44, 2.63) | 0.98 (0.82, 1.18) | 1.04 (0.94, 1.14) | 1.06 (1, 1.12) | 1.18 (1.11, 1.26) |
| Select non-traumatic causes | |||||||
| Diabetes | 1.2 (0.93, 1.55) | 0.99 (0.82, 1.19) | – | 0.64 (0.15, 2.64) | 1.78 (1.12, 2.83) | 1.09 (0.76, 1.56) | 0.93 (0.47, 1.85) |
| Circulatory | 1.09 (1.02, 1.16) | 1.54 (0.38, 6.26) | – | 1.11 (0.82, 1.5) | 0.94 (0.78, 1.13) | 1.03 (0.94, 1.14) | 1.18 (1.06, 1.3) |
| Cardiovascular | 1.07 (0.99, 1.15) | 0.93 (0.13, 6.73) | – | 1.06 (0.76, 1.48) | 0.99 (0.82, 1.2) | 0.98 (0.88, 1.1) | 1.17 (1.04, 1.31) |
| Ischemic | 1.06 (0.97, 1.16) | – | 1 (0.83, 1.2) | 1.1 (0.54, 2.23) | 1.09 (0.87, 1.37) | 0.98 (0.86, 1.12) | 1.12 (0.97, 1.3) |
| Cerebrovascular | 1.4 (1.15, 1.69) | – | – | 1.64 (0.66, 4.09) | 0.59 (0.26, 1.33) | 1.37 (1.08, 1.74) | 1.53 (1.17, 2.01) |
| Respiratory | 1.11 (0.99, 1.25) | – | – | 0.98 (0.4, 2.38) | 1.23 (0.88, 1.73) | 1.05 (0.89, 1.23) | 1.15 (0.95, 1.4) |
| Nephritis and nephrotic | 1.31 (0.91, 1.88) | 10.04 (1, 100.42)a | – | – | 0.99 (0.31, 3.15) | 1.22 (0.71, 2.11) | 1.48 (0.83, 2.63) |
| Acute renal failure | 1.08 (0.39, 2.94) | – | 1 (0.83, 1.2) | – | 3.03 (0.41, 22.34) | – | 1.27 (0.33, 4.95) |
| Mental disorders | 1.21 (0.97, 1.5) | 0.99 (0.82, 1.19) | 1 (0.83, 1.2) | 0.37 (0.05, 2.57) | 0.7 (0.26, 1.88) | 1.43 (1.02, 2.01) | 1.16 (0.86, 1.56) |
| Select traumatic causes | |||||||
| Natural heat and dehydration | 1.89 (0.82, 4.39) | – | – | – | 23.61 (4.45, 125.14)b | – | 1.6 (0.45, 5.69) |
| Accident | 1.19 (1.02, 1.39) | 0.58 (0.14, 2.51) | 1.24 (0.51, 3.02) | 1.16 (0.92, 1.46) | 1.14 (0.83, 1.55) | 1.43 (1.03, 1.98) | 1.17 (0.75, 1.84) |
| Suicide | 0.87 (0.66, 1.13) | 0.99 (0.82, 1.19) | – | 0.91 (0.63, 1.31) | 0.83 (0.51, 1.36) | 0.52 (0.22, 1.24) | 2.68 (0.93, 7.69) |
| Homicide | 0.83 (0.52, 1.34) | – | 1.76 (0.23, 13.34) | 0.9 (0.54, 1.52) | 0.44 (0.11, 1.81) | 1.74 (0.36, 8.29) | – |
Bolded relative risk values are significantly greater than 1 (p<0.05); “−” too few cases available to calculate
While statistically significant, the estimate is based on a small number of cases (four cases on non-heat days, one case on a heat day)
While statistically significant, the estimate is based on a small number of cases (five cases on non-heat days, three cases on a heat day)
Fig. 1.
Proportional cause-of-death burden in relation to total death, on days designated as heat days; size of bubbles represent proportion of mortality compared with all causes, while placement on the y-axis represents mortality relative risk estimate
When investigating mortality stratified by age, statistically significant increases in risk on a heat day compared with a non-heat day were found for: the 0–4 age group, nephritis and nephrotic syndromes (904 %); the 45–64 age group, diabetes (78 %) and natural heat exposure (2,261 %); the 65–84 age group, all-cause (6 %), non-traumatic (6 %), cerebrovascular (37 %), mental disorders (43 %), and accident (43 %); and the 85+ age group, all-cause (18 %), non-traumatic (18 %), circulatory (18 %), cardiovascular (17 %), and cerebrovascular (53 %). Relative risk estimates and 95 % confidence intervals for all-age groups and categories of death are reported in Table 4. It should be noted that both the 0–4 age group’s nephritis and nephrotic syndromes and the 45–64 age group’s natural heat exposure estimates are based on a small number of cases (1 and 3 heat-day cases, respectively) as reported in Table 4.
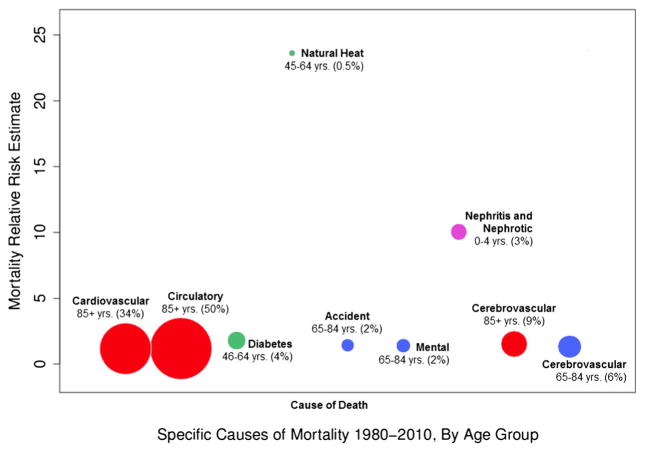
To achieve a better understanding of the relative burden of death for mortality stratified by age and cause of death, the proportional mortality is illustrated in Fig. 2. Statistically significant, age-adjusted cause-of-death categories run along the x-axis, while the corresponding relative risk estimates are reflected by the y-axis. Figure 2 illustrates the proportion of death for each age-adjusted cause, in relation to the age-adjusted, all-cause mortality on heat days (e.g., circulatory cause-of-death account for approximately 50 % of all deaths in the 85+ age group, on a heat day).
Fig. 2.
Proportional cause-of-death burden in relation to total death, on days designated as heat days, by statistically significant age category; size of bubbles represent proportion of age-adjusted mortality compared with all causes, while placement on the y-axis represents mortality relative risk estimate
Time series analysis
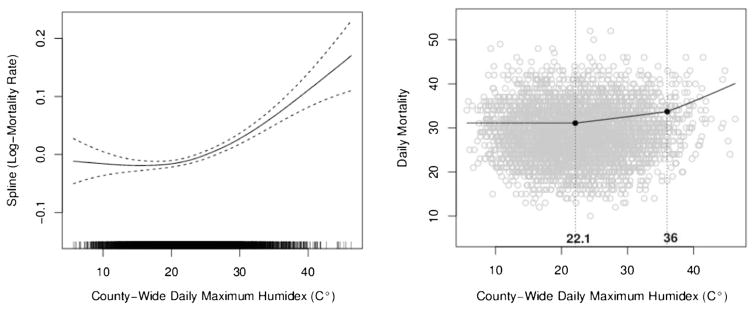
In King County, the time series analysis, modeled by a penalized cubic regression spline, results in a J-shaped curve. The relationship suggests an increased risk of mortality from exposure to humidex exceeding approximately 30 °C humidex. Improving interpretability, a piece-wise linear approximation, using two knots, was used to summarize heat effect on mortality, while a natural cubic spline (df=19) continued to model the temporal trend of mortality over 31 years. The piece-wise linear approximation’s first knot was set at the 50th percentile (22.1 °C), while the second knot was determined by increasing the model by 0.1 °C until the maximum likelihood was identified by the Akaike Information Criterion (AIC). The second knot, or optimal threshold, is just shy of the 99th percentile, at 36.0 °C (96.8 °F). Figure 3 illustrates the non-parametric spline model and corresponding piece-wise linear approximation for King County’s all-cause log-mortality rate and humidex relationship. During 1980–2010, King County experienced 117 days that exceeded the optimal threshold. The average maximum humidex on these exceedance days was 38.6 °C (101.4 °F).
Fig. 3.
Nonparametric spline model of all-cause log-mortality rate and humidex relationship; right, corresponding piece-wise linear approximation using two knots at 22.1 and 36.0 °C humidex
For all ages, all causes, we observed a 1.7 % increase in mortality per degree increase in county-wide daily maximum humidex above 36.0 °C. Statistically significant all-age results were also found for non-traumatic (2.1 %), circulatory (2 %), ischemic (2.5 %), cerebrovascular (6.2 %), and respiratory (4.4 %) causes of death. When investigating mortality stratified by age, statistically significant results were found for: the 15–44 age group, all-cause (−4.2 %) and accident (−10.2 %); the 45–64 age group, diabetes (14.2 %); the 65–84 age group, homicide (43.7 %); and the 85+ age group, all-cause (3.7 %), non-traumatic (3.8 %), circulatory (4.1 %), cardiovascular (4.3 %), ischemic (4.2 %), cerebrovascular (9.7 %), and suicide (43.4 %). Intensity estimates and 95 % confidence intervals for all-age groups and categories of death are reported in Table 5. It should be noted that both the 65–84 age group’s homicide and the 85+ age group’s suicide estimates are based on a small number of cases (3 and 4 heat-day cases, respectively) as reported in Table 5.
Table 5.
Time series analysis results: percentage (95 % CI) increase in mortality per degree increase in county-wide average daily maximum humidex (°C) above 36.0 °C, by age group and underlying cause of death
| All ages | 0–4 | 5–14 | 15–44 | 45–64 | 65–84 | 85+ | |
|---|---|---|---|---|---|---|---|
| All causes | 1.7 % (0.7 %, 2.7 %) | −5.9 % (−16.0 %, 5.4 %) | 4.3 % (−11.8 %, 3.4 %) | −4.2 % (−8.1 %, −0.1 %) | −0.3 % (−2.9 %, 2.3 %) | 1.0 % (−0.7 %, 2.6 %) | 3.7 % (1.8 %, 5.7 %) |
| Non-traumatic | 2.1 % (1.1 %, 3.2 %) | −4.3 % (−14.8 %, 7.5 %) | 12.7 % (−13.6 %, 47.0 %) | 1.7 % (−3.9 %, 7.6 %) | −0.3 % (−3.0 %, 2.5 %) | 0.9 % (−0.7 %, 2.6 %) | 3.8 % (1.9 %, 5.9 %) |
| Select non-traumatic causes | |||||||
| Diabetes | 2.5 % (−4.7 %, 10.3 %) | – | – | 13.3 % (−13.9 %, 49.1 %) | 14.2 % (2.2 %, 27.6 %) | −3.7 % (−13.4 %, 7.1 %) | −14.1 % (−31.2 %, 7.7 %) |
| Circulatory | 2.0 % (0.3 %, 3.7 %) | −11.7 % (−58.5 %, 90.7 %) | – | 0.0 % (−9.0 %, 10.0 %) | −1.0 % (−5.8 %, 4.1 %) | 0.6 % (−2.0 %, 3.3 %) | 4.1 % (1.3 %, 7.1 %) |
| Cardiovascular | 1.7 % (−0.2 %, 3.7 %) | −8.6 % (−63.5 %, 29.7 %) | – | −3.1 % (−13.1 %, 8.0 %) | 0.5 % (−4.6 %, 6.0 %) | 0.1 % (−3.0 %, 3.2 %) | 4.3 % (0.9 %, 7.7 %) |
| Ischemic | 2.5 % (0.1 %, 5.0 %) | – | – | 7.6 % (−13.5 %, 33.9 %) | 4.3 % (−1.3 %, 10.1 %) | 0.4 % (−2.9 %, 3.7 %) | 4.2 % (0.4 %, 8.2 %) |
| Cerebrovascular | 6.2 % (2.0 %, 10.6 %) | – | – | 1.6 % (−22.8 %, 33.6 %) | −6.5 % (−22.0 %, 12.1 %) | 3.2 % (−2.4 %, 9.2 %) | 9.7 % (3.8 %, 15.9 %) |
| Respiratory | 4.4 % (1.1 %, 7.7 %) | – | – | 10.7 % (−15.4 %, 44.9 %) | 5.4 % (−5.0 %, 16.8 %) | 3.1 % (−1.8 %, 8.3 %) | 3.6 % (−2.1 %, 9.6 %) |
| Nephritis and nephrotic | 7.7 % (−2.2 %, 18.7 %) | 18.3 % (−32.8 %, 108.2 %) | – | – | −8.0 % (−34.8 %, 29.8 %) | 5.5 % (−9.7 %, 23.2 %) | 15.7 % (−1.6 %, 36.1 %) |
| Acute renal failure | 10.9 % (−10.7 %, 37.8 %) | – | – | – | 29.1 % (−20.6 %, 110 %) | – | 14.2 % (−11.8 %, 47.8 %) |
| Mental disorders | −2.9 % (−9.3 %, 4.0 %) | – | – | −8.3 % (−35.8 %, 30.9 %) | −18.9 % (−39.4 %, 8.6 %) | 2.4 % (−6.8 %, 12.5 %) | −7.1 % (−14.7 %, 1.1 %) |
| Select traumatic causes | |||||||
| Natural heat and dehydration | 12.7 % (−6.6 %, 36.0 %) | – | – | – | 36.4 % (−24.6 %, 46.6 %) | – | −2.8 % (−47.5 %, 80.1 %) |
| Accident | −1.5 % (−5.8 %, 3.1 %) | −10.4 % (−42.9 %, 40.6 %) | 4.7 % (−16.6 %, 31.4 %) | −10.2 % (−16.8 %, −3.0 %) | 2.8 % (−6.3 %, 12.7 %) | 3.7 % (−6.1 %, 14.6 %) | −4.4 % (−17.1 %, 10.4 %) |
| Suicide | −7.0 % (−14.7 %, 1.4 %) | – | – | −7.8 % (−17.6 %, | −12.9 % (−27.0 %, 4.0 %) | −14.5 % (−35.0 %, 12.4 %) | 43.4 % (11.9 %, 83.8 %)a |
| Homicide | −7.4 % (−20.5 %, 7.9 %) | – | −10.7 % (−53.8 %, 72.5 %) | −12.6 % (−28.7 %, 7.2 %) | −3.1 % (−31.2 %, 36.4 %) | 43.7 % (0.6 %, 105.3 %)b | – |
Bolded time series estimates are significantly greater than 0 (p<0.05); “−” too few cases available to calculate; results have been rounded to one decimal place
While statistically significant, the estimate is based on a small number of cases (58 cases on days below optimal threshold, 4 cases on days exceeding optimal threshold)
While statistically significant, the estimate is based on a small number of cases (45 cases on days below optimal threshold, 3 cases on days exceeding optimal threshold)
Effect modification with individual-level characteristics
We did not find that the following individual-level characteristics altered the risk of dying on a heat day: gender, race, high school graduation, marital status, Hispanic origin, or whether or not tobacco use contributed to death. However, we did find that age statistically significantly increased the risk of dying on a heat day. In both the relative risk and time series analysis, we found the 85+ age group to experience the greatest risk for all causes of mortality.
Other heat effects on mortality
We did not find a statistically significant cool-down effect on mortality; the difference between minimum and maximum humidex on a given heat day did not significantly influence mortality rates. Likewise, we found no effect on mortality from duration of consecutive heat days above threshold. Additionally, we found no significant effect on the mortality rate from the lagged humidex, while the acute association between excess humidex above the upper threshold and mortality on the same day (Lag0) remained statistically significant. Lastly, mortality associated with the type of synoptic weather classification on a given heat day was explored. During the study period, there were 168 dry tropical (DT) and 40 moist tropical (MT) days classified for the Seattle/Tacoma station. The corresponding maximum humidex on these days averaged 33.7 °C. Seventy-three out of the 117 days that exceeded the lower 36.0 °C TSA threshold were classified as either DT or MT (64 DT and 9 MT) with the maximum humidex averaging 39.1 °C. The risk of all-age, all-cause mortality was 39.6 % higher per one degree above 36.0 °C on days classified as having either a moist or dry tropical weather type compared with other synoptic weather type classifications.
Discussion
This study characterized King County, Washington’s historic heat-mortality relationship using two different statistical methods. Our relative risk analysis quantified the excess mortality on a heat day compared with a non-heat day, while our time series analysis quantified the intensity effect of heat on mortality for each one degree increase in humidex over the threshold. We further characterized risk by age groups and subcategories of all-cause mortality. This study explored cool-down, duration, lag, and synoptic weather type effects on mortality, and it quantified effect modification from available individual-level characteristics. The results demonstrate that heat, expressed as humidex, is associated with increased mortality on heat days, and that the risk increases with heat’s intensity.
Our study design offers advantages over others, as it allows for direct comparison between two commonly used analyses. First, we are able to compare two ways of defining a heat day: a relative threshold calculated from a fixed percentile to an absolute threshold estimated using a fitted model. Our relative risk analysis uses the 99th percentile as the definition of an extreme-heat day; the threshold for this is 36.1 °C. In comparison, our time series threshold was calculated at 36.0 °C using a more complicated piece-wise linear approximation. From a practical standpoint, the similarity between the two thresholds offers support to choosing a fixed percentile as a simple way to define extreme heat. Second, our study design offers a more complete picture of regional heat effects. The relative risk analysis provides a robust analysis of heat’s overall contribution to excess mortality on heat days, while the time series analysis allows for a nuanced understanding of the effect of heat on mortality and the role of potential effect modifiers.
This study found a statistically significant increase in all-age, all-cause mortality with both analyses. The relative risk of death on a heat day was 10 % greater than on a non-heat day, with risk increasing 1.69 % for each degree increase in humidex above 36.0 °C. We also found a statistically significant increase in all-age, non-traumatic mortality for both analyses. The relative risk of death on a heat day was 10 % greater than on a non-heat day, with risk increasing 2.12 % for each degree increase in humidex above 36.0 °C. )Jackson et al. (2010) found a similar 10 % increase in mortality risks for the 65+ and 75+ age groups living in the Greater Seattle area. Comparatively, Medina-Ramón and Schwartz (2007) meta-analysis of 42 cities found a smaller, 3.85 %, increase in mortality on extreme-heat days (above the 99th percentile) compared with all other days, while their piece-wise linear approximation for heat intensity effects on total mortality found a 0.70 % increase in risk (lag 0) for each 1 °C above 17 °C.
When investigating a log-linear increase in mortality above an absolute threshold, our previous research using a shorter time frame (1980–2006) found similar threshold and intensity results; each one degree increase in humidex above 35.7 °C was associated with 1.8 % increase in non-traumatic causes of death (Isaksen et al. 2014). However, other US-based studies conducted in 9 California counties, 9 US cities, and 20 US cities have found smaller (1–3 %) increases in daily non-traumatic mortality per 10 °F increase in daily apparent temperature, equivalent to approximately a 0.2–0.5 % increase in morality per 1 °C increase (Basu et al. 2005, 2008; Zanobetti and Schwartz 2008). Compared with European results, similar magnitude, albeit lower thresholds, were found (1.8 and 3.1 % increases per 1 °C apparent temperature for thresholds of 23.3 and 29.4 °C in North-Continental and Mediterranean regions of Europe, respectively) (Baccini et al. 2008). The variability among studies further supports the importance of regional analysis.
Comparing results from the relative risk and time series analysis suggests that the modifying effect age has on mortality may be partly influenced by heat’s intensity. When stratifying cause-of-death categories by age group in the relative risk analysis, we found that heat’s overall effect does not exclusively impact the elderly (85+ age group). Statistically significant results were found in the 0–4 (nephritis and nephrotic), 45–64 (diabetes, and natural heat exposure), and 65–84 (all causes, non-traumatic, cerebrovascular, mental disorders, and accident) year-old age groups. However, when examining heat’s intensity effect using the time series analysis, we found that, with a few exceptions (15–44 all-cause and accident, 45–64 diabetes, and 65–84 homicide), heat’s intensity almost exclusively affects the 85+ age group (all-cause, non-traumatic, circulatory, cardiovascular, ischemic, cerebrovascular, and suicide). Isaksen et al. (2014) found similar statistically significant age-stratified results for the 85+ age group when comparing heat’s intensity effect on circulatory (4.8 %) and cardiovascular (4.22 %) causes of death in King County.
Results from both analyses suggest that a vulnerable population of younger diabetic patients exists. For the 45–64-year-old age group, we found that the relative risk of death from diabetes on a heat day was 78 % greater than on a non-heat day, with risk increasing 14.2 % for each degree increase in humidex above 36.0 °C. In comparison, Schuman (1972) found a 117 % increase in diabetic-related mortality during a New York heat wave, while Basagaña et al. (2011) observed a 20 % increase in mortality risk for diabetes on extreme-heat days in the Catalonia region of Spain. Schwartz (2005) found that the relative odds of death, on a 99th percentile day, increased 17 % for those persons previously hospitalized for diabetes. One possible explanation for the increased diabetic mortality risk is suggested by Akanji and Oputa’s (1991) study findings where both non-diabetic and diabetic participants’ plasma glucose levels significantly increased with a 10 °C increase in ambient temperature. Given that an estimated 8 % of 45–64-year olds and 15 % of 65+-year olds living in King County have diabetes, our findings are an important consideration for outreach and prevention programs (Public Health-Seattle and King County 2013).
This study also highlights the vulnerability of older age groups to circulatory deaths and its subcategories, cardiovascular and cerebrovascular causes. Similarly, Jackson et al. (2010) found in the Greater Seattle area that the highest elevated risk for circulatory mortality on a heat day belonged to the 65+ (30 %) and 85+ (50 %) age groups. Isaksen et al. (2014) found that, in King County, the 85+ age group’s risk for circulatory mortality increased 4.8 % for each degree above 35.7 °C humidex. Studies from other geographic locations have also shown an increased risk in heat-related mortality for the elderly (Basu et al. 2008; Ishigami et al. 2008). Age-related vulnerability has been attributed to changes in the body’s natural homeostatic mechanisms, greater likelihood of taking medications that inhibit the body’s thermo-regulation, being homebound, living alone, and not being able to care for one’s self (Brown and Walker 2008; Kovats and Hajat 2008; Naughton 2002).
Aside from age, this study did not find that the available individual-level covariates modified the effect of heat on mortality. However, other studies have found that individuals of Black race or non-White race (O’Neill et al. 2003; O’Neill et al. 2005), those with less education, and those who are socially isolated are more vulnerable to heat-related death (Medina-Ramón et al. 2006; O’Neill et al. 2003; Naughton 2002). It is possible that other individual-level characteristics such as social isolation, socio-economic status, and educational attainment, not collected through death certificate data, are more relevant predictors of vulnerability to heat-related mortality than the characteristics we were able to consider. It is also possible that neighborhood-level characteristics that were not measured in the study, such as the presence of urban heat islands, design of spaces to include shade, and social cohesion, could have greater influence on heat-related mortality than individual characteristics.
An alternative explanation for the lack of identified vulnerable populations in this study could derive from our region’s low prevalence of air conditioning. Hamlet et al. (2010) have calculated King County’s air conditioning penetration rate at approximately 8 %. Our unpublished research for Pierce County (King’s neighbor immediately to the South) found 10 % of all dwelling-occupied parcels had air conditioning as of 2009, quite similar to the Hamlet et al. report. The availability and use of air conditioning is considered the strongest factor in preventing heat-related mortality (O’Neill et al. 2003; Davis et al. 2004; Naughton 2002; Curriero et al. 2002). It is possible that as our region warms and the availability and use of air conditioning increases, vulnerable subpopulations may be detected. An additional explanation for the lack of identified vulnerable populations in this study could be the different geographical scale between our study and those that have identified vulnerable populations. Studies that have found effect modification within subpopulations (O’Neill et al. 2003; O’Neill et al. 2005; Medina-Ramón et al. 2006; Naughton 2002) were confined to either cities or urban communities, whereas our study looked at heat’s influence on an entire county. This larger geographical scale produces different demographics and affects our power to detect differences across racial/ethnic or SES subpopulations (e.g., according to the 2010 census, King County is reportedly 70.8 % White, 6.6 % African American, 9.3 % Hispanic, 11.5 % below poverty level, and 92.1 % with high school education or more).
This study found that the same-day humidex exposure had the strongest association with mortality, and that there was no evidence of a lag or cool-down effect. These findings are similar to those of other studies in which either the same-day apparent temperature (Gasparrini and Armstrong 2011; Anderson and Bell 2009; Zanobetti and Schwartz 2008) or recent lags of 1–3 days were found to be most relevant to the heat-mortality relationship (Basagaña et al. 2011; Basu et al. 2008; Curriero et al. 2002). This study did not find a duration effect, the number of consecutive days exceeding a threshold affected mortality rates. Heat events of long duration are rare in King County. It is predicted that the duration of events will lengthen with climate change, and therefore, it is possible that in the future we may detect a difference in mortality risk with longer events. This study found that days classified as having either a moist or dry tropical synoptic weather classification were associated with a significant increased risk of mortality than days that were not classified as moist or dry tropical. Our research supports Kalkstein and Greene (1997) and Kalkstein et al. (2011) findings of increased mortality on days with oppressively hot air masses.
Limitations of this study include possible exposure misclassification and inappropriate geographical boundary selection. Our study uses an average daily county-wide maximum humidex value to estimate heat exposure, which may result in exposure misclassification when a disproportionate number of cases are below or above the average value. Improved exposure assessment could be obtained by using a population-weighted temperature value, or by assigning each case a maximum humidex value from the closest meteorological grid center point. Data regarding access to air conditioning and behavioral/lifestyle choices would further refine heat exposure. Personal monitoring of time-activity patterns from a representative sample of vulnerable populations could help clarify these factors, as was done in a previous study of elderly individuals (Basu and Samet 2002). In this study, humidex was calculated using an average daily relative humidity. It is possible that by using the average daily relative humidity, our model threshold may be higher than the true heat-health threshold. However, even if our metric is biased high, it is still below the current National Weather Service warning criteria for this region.
This study used political jurisdictions (county boundary) to assess the heat-mortality relationship. This geographical unit of analysis may not accurately reflect how the effects of heat on mortality vary spatially. An alternative method, and area for future research, would be to combine populations that experience similar climate zones and, therefore, should have similar levels of acclimatization. Combining populations into climate zones may also reduce type II error, by ensuring adequate power, allowing the examination of heat-related health effects in rural areas.
This study did not correct for multiple comparisons. A type 1 error may occur when numerous subgroups are analyzed for effect difference. The more comparisons that are analyzed, the more opportunity there is to identify, by chance, a result that appears significant, even when no statistically significant difference exists. A multiple testing correction, such as Bonferroni, could be applied to our analyses (56). However, conventional multiple testing correction methods can be overly conservative, resulting in an increase in false negative results. Instead, we progressively analyzed our data, looking at overall, all-age results prior to analyzing subcategories of cause and age. Our analyzed subcategories were chosen a priori based on previous where research conducted by our group, findings from the literature and observations from the practice community. The statistically significant results were then examined for expected dose–response patterns, concurrence with existing literature, biologic plausibility, and influence of small counts. Results were flagged in data presentation and discussion when found to depend on a small number of outcomes (N<20). All other results have been provided, along with confidence intervals.
This study did not adjust for air pollution, as our primary focus was the total effect of heat on mortality rather than the direct effect. A recent commentary by Buckley et al. (2014) noted that adjustment for air pollution can lead to biased estimates of effects due to heat, and that such adjustments should not be conducted without a clear rationale. Studies that have controlled for air pollution, or that have analyzed effect modification from air pollutants, have found that the association between heat and mortality persists (Anderson and Bell 2009; Basu et al. 2008; Zanobetti and Schwartz 2008). For example, Anderson and Bell’s (2009) meta-analysis observed only slight decreases in mortality risk after adjusting for Ozone or PM10. In their subsequent analysis (Anderson and Bell 2011) of the same communities, they chose not to control for air pollution, citing temperature’s robust effects on mortality. Similar findings of no significant confounding or effect modification from air pollutants were reported by Basu et al. (2008) and Zanobetti and Schwartz (2008) in their analysis of nine California counties and nine US cities, respectively.
In conclusion, this study characterized King County, Washington’s historic heat-mortality relationship using two different statistical methods. The results demonstrate that heat, expressed as humidex, is associated with increased mortality on heat days, and that the risk increases with heat’s intensity. When stratifying by age and cause of death, younger age groups were at an increased risk of death for several causes of death, particularly diabetes. Additionally, we found that heat’s intensity almost exclusively affected the 85+ age group. While individual-level characteristics (age being an exception) and other heat effects (cool-down, duration, lag) were not found to affect mortality rates, the synoptic weather classification was found to modify risks.
Future research is needed to validate the methods used to model our heat-mortality relationship, as our piece-wise linear model fits linear slopes to an otherwise non-linear relationship. Improving heat exposure assessment is another area where additional research would improve the understanding of our region’s heat-mortality relationship. Our findings warrant additional investigation into the role heat exposure plays in diabetic patient health and care. Lastly, a better understanding of the full range of effects that air mass type and other meteorological metrics have on mortality would further elucidate ways to improve public health preparedness and response measures.
Acknowledgments
Thank you to Matt Stumbaugh, Eric Salathé, and Alan Hamlet, with the University of Washington’s Climate Impacts Group, for providing the meteorological dataset as well as technical support. This work was supported, in part, by funding from the University of Washington’s Department of Environmental and Occupational Health Sciences Initiative Grant, the Centers for Disease Control and Prevention Cooperative Agreement, “Confronting the Health Risks of Climate Change” (1 U01 EH 000400-01), and the University of Washington Biostatistics, Epidemiologic and Bioinformatic Training in Environmental Health (BEBTEH) Training Grant - sponsored by the National Institute of Environmental Health Sciences (T32ES015459).
Footnotes
Ethical standards This study complies with the Washington State Department of Health and University of Washington’s ethical standards. Data analyses were conducted after proper and appropriate human subjects approval was obtained from the Washington State Department of Social and Health Services IRB.
References
- Akanji AO, Oputa RN. The effect of ambient temperature on glucose tolerance. Diabet Med. 1991;8(10):946–948. doi: 10.1111/j.1464-5491.1991.tb01534.x. [DOI] [PubMed] [Google Scholar]
- Anderson BG, Bell ML. Heat waves in the United States: mortality risk during heat waves and effect modification by heat wave characteristics in 43 U.S. communities. Environ Health Perspect. 2011;119(2):210–218. doi: 10.1289/ehp.1002313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson BG, Bell ML. Weather-related mortality: how heat, cold, and heat waves affect mortality in the United States. Epidemiology. 2009;20(2):205–13. doi: 10.1097/EDE.0b013e318190ee08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong B. Models for the relationship between ambient temperature and daily mortality. Epidemiology. 2006;17(6):624–631. doi: 10.1097/01.ede.0000239732.50999.8f. [DOI] [PubMed] [Google Scholar]
- Baccini M, Biggeri A, Accetta G, Kosatsky T, Katsouyanni K, Analitis A, Anderson HR, Michelozzi P. Heat effects on mortality in 15 European cities. Epidemiology. 2008;19(5):711–9. doi: 10.1097/EDE.0b013e318176bfcd. [DOI] [PubMed] [Google Scholar]
- Barnett AG, Tong S, Clements ACA. What measure of temperature is the best predictor of mortality? Environ Res. 2010;110(6):604–611. doi: 10.1016/j.envres.2010.05.006. [DOI] [PubMed] [Google Scholar]
- Basagaña X, Sartini C, Barrera-Gómez J, Dadvand P, Cunillera J, Ostro B, Sunyer J, Medina-Ramón M. Heat waves and cause-specific mortality at all ages. Epidemiology. 2011;22(6):765–72. doi: 10.1097/EDE.0b013e31823031c5. [DOI] [PubMed] [Google Scholar]
- Basu R, Samet JM. An exposure assessment study of ambient heat exposure in an elderly population in Baltimore, Maryland. Environ Health Perspect. 2002;110(12):1219–1224. doi: 10.1289/ehp.021101219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basu R, Dominici F, Samet JM. Temperature and mortality among the elderly in the United States. Epidemiology. 2005;16(1):58–66. doi: 10.1097/01.ede.0000147117.88386.fe. [DOI] [PubMed] [Google Scholar]
- Basu R, Feng WY, Ostro BD. Characterizing temperature and mortality in nine California counties. Epidemiology. 2008;19(1):138–45. doi: 10.1097/EDE.0b013e31815c1da7. [DOI] [PubMed] [Google Scholar]
- Brown S, Walker G. Understanding heat wave vulnerability in nursing and residential homes. Build Res Inf. 2008;36(4):363–372. [Google Scholar]
- Buckley JP, Samet JM, Richardson DB. Commentary: does air pollution confound studies of temperature? Epidemiology. 2014;25(2):242–5. doi: 10.1097/EDE.0000000000000051. [DOI] [PubMed] [Google Scholar]
- Canadian Centre for Occupational Health and Safety. [Accessed 10 Nov. 2011];Humidex rating and work. 2011 http://www.ccohs.ca/oshanswers/phys_agents/humidex.html.
- Cheng CS, Campbell M, Li Q, Li G, Auld H, Day N, Pengelly D, Gingrich S, Klaassen J, MacIver D, Comer N, Mao Y, Thompson W, Lin H. Toronto, Canada: Health Canada, Health Policy Research Program; 2005. [Accessed 10 Nov. 2013]. Differential and combined impacts of winter and summer weather and air pollution due to global warming on human mortality in South-central Canada (6795-15-2001/4400011) http://www.toronto.ca/health/hphe/pdf/weather_air_pollution_impacts.pdf. [Google Scholar]
- Curriero FC, Heiner KS, Samet JM, Zeger SL, Strug L, Patz JA. Temperature and mortality in 11 cities of the eastern United States. Am J Epidemiol. 2002;155(1):80–7. doi: 10.1093/aje/155.1.80. [DOI] [PubMed] [Google Scholar]
- Daly C, Halbleib M, Smith JI, Gibson WP, Doggett MK, Taylor GH, Curtis J, Pasteris PP. Physiographically sensitive mapping of climatological temperature and precipitation across the conterminous United States. Int J Climatol J R Meteorol Soc. 2008;28(15):2031–2064. [Google Scholar]
- Davis RE, Knappenberger PC, Michaels PJ, Novicoff WM. Seasonality of climate-human mortality relationships in US cities and impacts of climate change. Clim Res. 2004;26(1):61. [Google Scholar]
- D’Ippoliti D, Michelozzi P, Marino C, De’Donato F, Menne B, Katsouyanni K, Kirchmayer U, Perucci CA. The impact of heat waves on mortality in 9 European cities: results from the EuroHEAT project. Environ Heal. 2010;9(1):1–9. doi: 10.1186/1476-069X-9-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasparrini A, Armstrong B. The impact of heat waves on mortality. Epidemiology. 2011;22(1):68–73. doi: 10.1097/EDE.0b013e3181fdcd99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasparrini A. Distributed lag linear and non-linear models in R: the package dlnm. [Accessed 15 Jan. 2014];J Stat Softw. 2011 43(8):1–20. http://www.jstatsoft.org/v43/i08/ [PMC free article] [PubMed] [Google Scholar]
- Hamlet AF, Lee S-Y, Mickelson KEB, Elsner MM. Effects of projected climate change on energy supply and demand in the Pacific Northwest and Washington State. Clim Chang. 2010;102:103–128. [Google Scholar]
- Harlan SL, Brazel AJ, Prashad L, Stefanov WL, Larsen L. Neighborhood microclimates and vulnerability to heat stress. Soc Sci Med. 2006;63(11):2847–2863. doi: 10.1016/j.socscimed.2006.07.030. [DOI] [PubMed] [Google Scholar]
- Isaksen TB, Yost M, Hom E, Fenske R. Projected health impacts of heat events in Washington State associated with climate change. Rev Environ Health. 2014;29:1–2. doi: 10.1515/reveh-2014-0029. [DOI] [PubMed] [Google Scholar]
- Ishigami A, Hajat S, Kovats RS, Bisanti L, Rognoni M, Russo A, Paldy A. An ecological time-series study of heat-related mortality in three European cities. Environ Health Glob Access Sci Source. 2008;7:5. doi: 10.1186/1476-069X-7-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson JE, Yost MG, Lamb BK, Lamb BK, Lamb BK, Chung SH, Rosenblatt RA, Fenske RA. Public health impacts of climate change in Washington State: projected mortality risks due to heat events and air pollution. Clim Chang. 2010;102:1–2. [Google Scholar]
- Jones TS, Liang AP, Kilbourne EM, Griffin MR, Patriarca PA, Wassilak SG, Mullan RJ, Thacker SB. Morbidity and mortality associated with the July 1980 heat wave in St Louis and Kansas City, MO. JAMA J Am Med Assoc. 1982;247(24):3327–31. [PubMed] [Google Scholar]
- Kaiser R, Rubin CH, Henderson AK, Wolfe MI, Kieszak S, Parrott CL, Adcock M. Heat-related death and mental illness during the 1999 Cincinnati heat wave. Am J Forensic Med Pathol. 2001;22(3):303–7. doi: 10.1097/00000433-200109000-00022. [DOI] [PubMed] [Google Scholar]
- Kalkstein LS, Greene S, Mills DM, Samenow J. An evaluation of the progress in reducing heat-related human mortality in major U.S. cities. Nat Hazards. 2011;56(1):113–129. [Google Scholar]
- Kalkstein LS, Greene JS. An evaluation of climate/mortality relationships in large U.S. cities and the possible impacts of a climate change. Environ Health Perspect. 1997;105(1):84–93. doi: 10.1289/ehp.9710584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H, Ha J-S, Park J. High temperature, heat index, and mortality in 6 major cities in South Korea. Arch Environ Occup Health. 2006;61(6):265–270. doi: 10.3200/AEOH.61.6.265-270. [DOI] [PubMed] [Google Scholar]
- King County. [Accessed on 23 Sept. 2013];About King County and its Government. Updated December 21, 2012. http://www.kingcounty.gov/About.aspx.
- Klinenberg E. Heat wave: a social autopsy of disaster in Chicago. University of Chicago Press; Chicago: 2002. [DOI] [PubMed] [Google Scholar]
- Kovats RS, Hajat S. Heat stress and public health: a critical review. Annu Rev Public Health. 2008;29:41–55. doi: 10.1146/annurev.publhealth.29.020907.090843. [DOI] [PubMed] [Google Scholar]
- Masterton JM, Richardson FA. Humidex; a method of quantifying human discomfort due to excessive heat and humidity. Environment Canada, Atmospheric Environment; Downsview, Ont: 1979. [Google Scholar]
- Maurer EP, Wood AW, Adam JC, Lettenmaier DP, Nijssen B. A long-term hydrologically based data set of land surface fluxes and states for the conterminous United States. J Clim. 2002;15:3237–325. [Google Scholar]
- Medina-Ramón M, Schwartz J. Temperature, temperature extremes, and mortality: a study of acclimatisation and effect modification in 50 US cities. Occup Environ Med. 2007;64(12):827–33. doi: 10.1136/oem.2007.033175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina-Ramón M, Zanobetti A, Cavanagh DP, Schwartz J. Extreme temperatures and mortality: assessing effect modification by personal characteristics and specific cause of death in a multi-city case-only analysis. Environ Health Perspect. 2006;114(9):1331–1336. doi: 10.1289/ehp.9074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naughton M. Heat-related mortality during a 1999 heat wave in Chicago. Am J Prev Med. 2002;22(4):221–227. doi: 10.1016/s0749-3797(02)00421-x. [DOI] [PubMed] [Google Scholar]
- NOAA Satellite and Information Service. [Accessed 20 Nov. 2013];Global historical climatology network - daily. 2009 http://www.ncdc.noaa.gov/oa/climate/ghcn-daily/
- O’Neill MS, Zanobetti A, Schwartz J. Disparities by race in heat-related mortality in four US cities: the role of air conditioning prevalence. J Urban Health Bull N Y Acad Med. 2005;82(2):191–7. doi: 10.1093/jurban/jti043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Neill MS, Zanobetti A, Schwartz J. Modifiers of the temperature and mortality association in seven US cities. Am J Epidemiol. 2003;157(12):1074–82. doi: 10.1093/aje/kwg096. [DOI] [PubMed] [Google Scholar]
- PRISM Climate Group, Oregon State U. PRISM climate group, Oregon State U. N.p., n.d. Web. 15 Feb. 2014 Public Health - Seattle & King County (2013) Indicator: diabetes prevalence, King County.
- Public Health - Seattle King County. [Accessed 13 Jan. 2014]; http://www.kingcounty.gov/healthservices/health/data/indicators/HealthOutcomesDiabetesPrevalence.aspx.
- R Core Team. R: A language and environment for statistical computing. R foundation for statistical computing; Vienna, Austria: 2012. [Accessed 13 Jan. 2014]. http://www.r-project.org/ [Google Scholar]
- Schuman SH. Patterns of urban heat-wave deaths and implications for prevention: data from New York and St. Louis during July, 1966. Environ Res. 1972;5(1):59–75. doi: 10.1016/0013-9351(72)90020-5. [DOI] [PubMed] [Google Scholar]
- Schwartz J. Who is sensitive to extremes of temperature? A case-only analysis. Epidemiology. 2005;16(1):67–7. doi: 10.1097/01.ede.0000147114.25957.71. [DOI] [PubMed] [Google Scholar]
- Sheridan S. Spatial synoptic classification. Kent State University. Department of Geography; 2013. [Accessed 10 Nov. 2013]. http://sheridan.geog.kent.edu/ssc.html. [Google Scholar]
- Sheridan SC, Kalkstein AJ, Kalkstein LS. Trends in heat-related mortality in the United States, 1975–2004. Nat Hazards. 2009;50(1):145–160. [Google Scholar]
- Smoyer KE. Putting risk in its place: methodological considerations for investigating extreme event health risk. Soc Sci Med. 1998;47(11):1809–1824. doi: 10.1016/s0277-9536(98)00237-8. [DOI] [PubMed] [Google Scholar]
- Stone B, Hess JJ, Frumkin H. Urban form and extreme heat events: are sprawling cities more vulnerable to climate change than compact cities? Environ Health Perspect. 2010;118(10):1425–1428. doi: 10.1289/ehp.0901879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Washington State Office of Financial Management. [Accessed 13 Sept. 2013];King County census data. 2012 http://www.ofm.wa.gov/localdata/king.asp.
- Whitman S, Good G, Donoghue ER, Benbow N, Shou W, Mou S. Mortality in Chicago attributed to the July 1995 heat wave. Am J Public Health. 1997;87(9):1515–8. doi: 10.2105/ajph.87.9.1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yardley J, Sigal RJ, Kenny GP Special Issue on the Politics and Policy of Carbon Capture and Storage. Heat health planning: the importance of social and community factors. Glob Environ Chang. 2011;21(2):670–679. [Google Scholar]
- Zanobetti A, Schwartz J. Temperature and mortality in nine US cities. Epidemiology. 2008;19(4):563–70. doi: 10.1097/EDE.0b013e31816d652d. [DOI] [PMC free article] [PubMed] [Google Scholar]