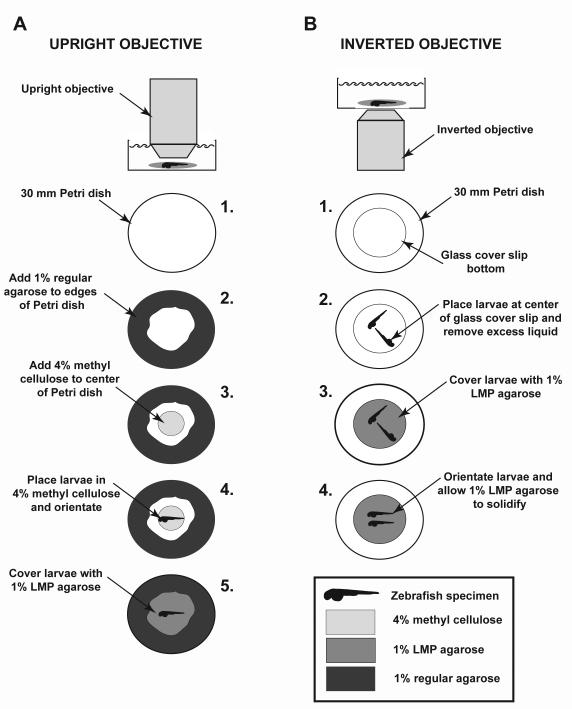
Fig. 6.
Schematic illustrating stable mounting procedures for regular and inverted microscopes. (A) When imaging using an upright objective, a 60-mm Petri dish is used (step 1), and 1% regular agarose is used to fill in around the edges of the Petri dish (step 2). Four percent methyl cellulose is then placed in center of Petri dish (step 3), before the anesthetized animal is placed in 4% methyl cellulose and oriented (step 4). Once specimen is correctly oriented, it is covered with 1% LMP agarose and allowed to solidify (step 5). (B) For imaging using an inverted objective, a 60-mm Petri dish with a fitted glass coverslip as a base is used (step 1). Anesthetized zebrafish are place onto the glass coverslip base (step 2) and excess liquid is removed. The specimen is then covered with 1% LMP agarose (step 3) and quickly oriented while agarose is solidifying (step 4). For both methods, 2 mL of system water, containing 1× tricaine, is added to specimen. When undertaking imaging using the upright objective method, be sure to use a water dipping objective.