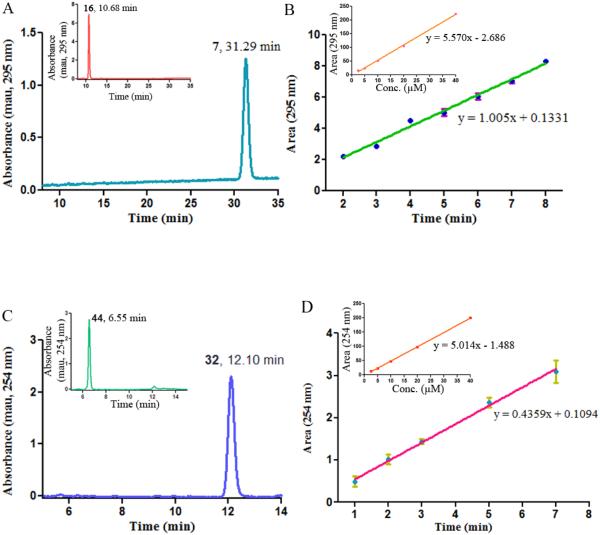
Figure 3.
Cleavage of the glycine conjugates of 2' CA4-amine 16 and the amino dihydronaphthalene 44 by LAP. (A) Prodrug 16 was treated with 0.5 units of LAP for 40 h. A single peak corresponding to the product 2' CA4 amine 7 (tR = 31.29 min) was observed (HPLC chromatogram mobile phase: 26% acetonitrile/74% water containing 0.05% TFA). Inset: Control, compound 16 was incubated for 40 h without LAP. (B) Rate study for the cleavage of prodrug 16 to form 2' CA4 amine 7. Inset: Calibration curve for 2' CA4 monoamine 7. (C) Prodrug 44 was treated with 0.05 units of LAP for 2 h. A single peak corresponding to the product amino dihydronaphthalene 32 (tR = 12.10 min) was observed (HPLC chromatogram mobile phase: 28% acetonitrile/72% water containing 0.05% TFA). Inset: Control, compound 44 was incubated for 2 h without LAP. (D) Rate study for the cleavage of prodrug 44 to form amino dihydronaphthalene 32. Inset: Calibration curve for amino dihydronaphthalene 32