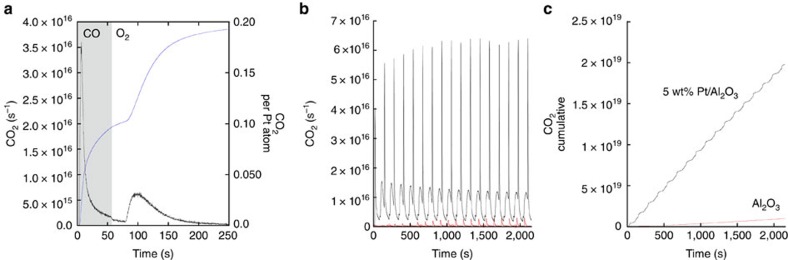
Figure 1. Periodic redox operation of the 5 wt% Pt/Al2O3 catalyst at 298 K.
(a) Transient evolution of CO2 observed during exposure of a pre-reduced Type-94 (Johnson Matthey) 5 wt% Pt/Al2O3 catalyst to 5 vol% CO/Ar (shaded area), followed by a switch to 21 vol% O2/He at 298 K. The left-hand axis reports the evolution of CO2 in terms of molecules per second (black), the right-hand axis shows the cumulative CO2 production as a fraction of the total number of Pt atoms in the catalyst bed (blue). (b) Repeated cycles of a similar (shorter oxidizing cycle) experiment shown in a: black=5 wt% Pt/Al2O3; red=Al2O3. (c) Cumulative CO2 (molecules) production during the experiment shown in b.