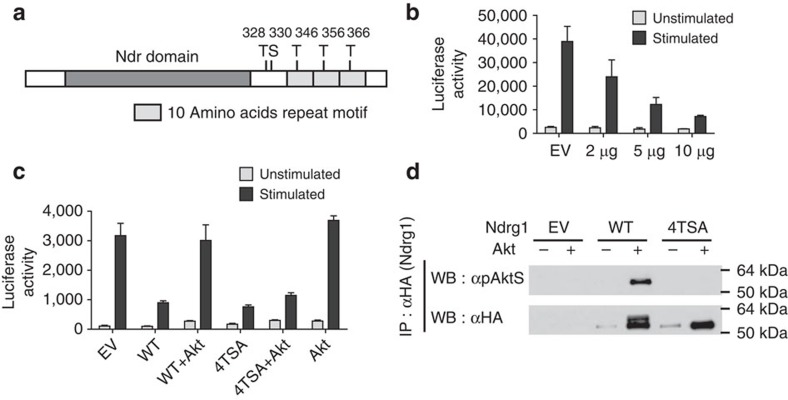
Figure 3. Inhibitory activity of Ndrg1 is abrogated by Akt-dependent phosphorylation.
(a) Molecular structure of Ndrg1. T, threonine; S, serine. The numbers represent the amino acid location of the threonine or serine residues. The size of each domain or motif is not to scale. (b,c) Jurkat cells were transfected with Ndrg1 and/or Akt plasmids along with an IL-2 promoter luciferase plasmid. Next, the cells were stimulated with anti-CD3 plus anti-CD28 for 6 h and luciferase activity of cell extracts was measured as described in Methods. (b) Dose–response inhibition of IL-2 transcription by Ndrg1 overexpression in Jurkat cells. μg, amount of Ndrg1 plasmid used for transfection; EV, the empty vector. (c) Co-transfection of Akt and Ndrg1 plasmids abrogates Ndrg1-mediated inhibition of IL-2 transcription, whereas co-transfection of the 4TSA mutant and Akt does not abrogate 4TSA-mediated inhibition of IL-2 transcription. WT, wild-type Ndrg1; 4TSA, T346A+T356A+T366A+T328A+S330A. (d) Akt-dependent phosphorylation of the wild-type Ndrg1 and not the 4TSA mutant. Jurkat cells were transfected with HA-tagged wild-type Ndrg1 or 4TSA mutant plus Akt plasmids. Overexpressed Ndrg1 proteins were immunoprecipitated with anti-HA and immunoblotted with α-pAktS or anti-HA. α-pAktS reacts with phosphorylated serine- or threonine-containing substrate peptides of Akt26. IP, immunoprecipitation; WB, western blotting. Results are representative of two (d), three (b) or four (c) independent experiments; error bars, s.d. for quadruplicates of each sample.