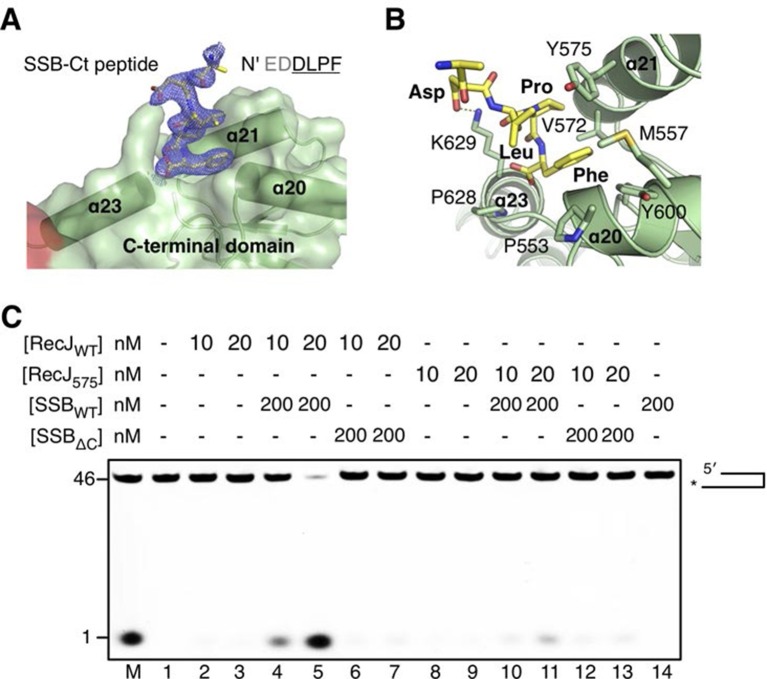
Figure 6. The C-terminal domain interacts with the SSB-Ct.
(A) SSB-Ct binding pocket. SSB-Ct is shown as stick. The electron density of SSB-Ct is shown in blue with the refined 2Fo-Fc map contoured at 1σ. (B) Interactions of the SSB-Ct and C-terminal domain. Both SSB-Ct (yellow) and residues involved in SSB-Ct interactions (green) are shown as sticks. The ionic bond between the Asp298 of SSB-Ct and Lys629 of drRecJ is indicated by a yellow dashed line. (C) SSB enhances wild-type drRecJ degradation. Y575A mutant drRecJ (RecJ575), wild-type drSSB (SSBWT) and drSSB lacking eight C-terminal residues (SSBΔC) were purified to perform the nuclease assays. For the reaction, DNA with a 3´-ssDNA overhang was pre-incubated with 200 nM SSBWT or SSBΔC and treated with drRecJ or RecJ575 (5 and 20 nM) (see Materials and methods).
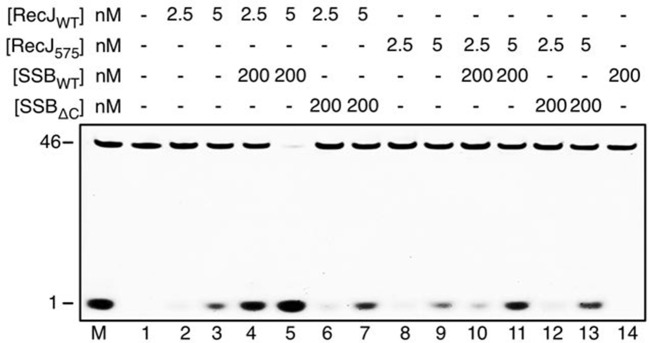
Figure 6—figure supplement 1. SSB enhances wild-type drRecJ degradation on ssDNA.
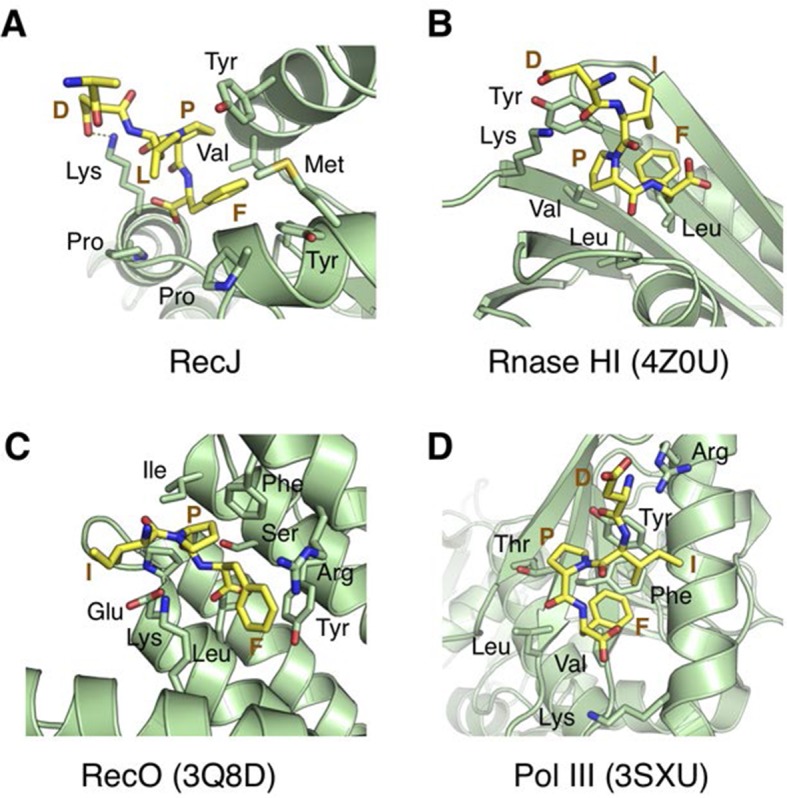
Figure 6—figure supplement 2. Structural comparison of the SSB-Ct binding pockets of RecJ, Rnase HI (4Z0U), RecO (3Q8D) and Pol III (3SXU).