Abstract
Elicitation effect of silver nano particles (AgNPs) and triggering of defence system by production of hydrogen peroxide (H2O2) as a signaling molecule in the regulation of the activity of stress-related enzymes and production of Taxol was evaluated in suspension- cultured hazel cells (Corylus avellana L.). The cells were treated with different concentrations of AgNPs (0, 2.5, 5, and 10 ppm), in their logarithmic growth phase (d7) and were harvested after 1 week. Treatment of hazel cells with AgNPs decreased the viability of the cells. Also the results showed that while the activity of certain radical scavenging enzymes in particular of catalase and peroxidase increased by 2.5 and 5 ppm AgNPs, the activity of superoxide dismutase decreased in these treatments. The highest activity of ascorbate peroxidase was observed in 10 ppm AgNPs treatments. This treatment also showed the highest contents of H2O2 and phenolic compounds, as well as the highest activity of phenylalanine ammonialyase. According to the results, 5 ppm AgNPs was the best concentration for elicitation of hazel cells to produce efficient amounts of H2O2 in order for stimulation of antioxidant defence system, production of Taxol at the highest capacity of the cells, meanwhile reserving their viability.
Electronic supplementary material
The online version of this article (doi:10.1007/s10616-014-9808-y) contains supplementary material, which is available to authorized users.
Keywords: AgNPs, Antioxidant activity, Corylus avellana L., Taxol
Introduction
Among noble-metal nanomaterials, silver nanoparticles (AgNPs) have received considerable attentions due to their attractive physicochemical properties. It is well known that silver in various chemical forms has strong toxicity to a wide range of microorganisms. In particular, AgNPs have been shown to be a promising antimicrobial material (Sondi and Salopek-Sondi 2004). The larger surface area of silver nanoparticles can improve their antibacterial effectiveness against 150 types of microbes. Due to antimicrobial activity, AgNPs have been applied in order to extent vase life of cut flowers or stored green vegetables (An et al. 2008; Lü et al. 2010). Studies on the effects of AgNPs on animal cells have suggested the generation of reactive oxygen species (ROS) and oxidative stress as one of the more important mechanisms of toxicity related to nanoparticle exposure (Nel et al. 2006; Kim et al. 2009; Ahmed et al. 2010). A dose-related decrease in the activity of antioxidant enzymes in the liver, as well as dose-dependent increases in glutathione depletion and lipid peroxidation in the liver and gills of Oryzias latipes after long exposure to AgNPs (Wu and Zhou 2013). Few studies however examined such a hypothesis on plant cells. Increase of membrane lipid peroxidation and suppression of radical scavenging capacity by application of higher concentrations of AgNPs on Artemisia annua have been recently reported (Ghanati and Bakhtiarian 2013).
Depending on their concentration in plants, ROS are well recognized for playing a dual role as both deleterious and beneficial species. At high concentration ROS cause damage to biomolecules, whereas at low/moderate concentration act as second messenger in intracellular signaling cascades that mediate several responses in plant cells (Dat et al. 2000). Majority of biologically active compounds of medicinal plants are defensive metabolites and can be induced by chemical elicitors. Therefore, it can be postulated that AgNPs function as elicitors which produce ROS thereby increase secondary metabolites. Elicitor potential of AgNPs to increase production of Taxol was evaluated in suspension-cultured hazel cells.
Materials and methods
Culture condition and AgNPs treatment
Suspension cultured hazel (Corylus avellana L.) cells (established by Rezaei et al. 2011) grown in a modified MS liquid medium without glycine and supplemented with 3 mg L−1 α-naphthalene acetic acid (NAA) and 3 mg L−1 indole-3-acetic acid (IAA) were used. Unless otherwise stated, all chemicals and reagents were purchased from Sigma-Aldrich (St. Louis, MO, USA). The cells were maintained at 25 °C in dark with shaking at 110 rpm and were sub cultured every 7 days.
Seven days old cells (logarithmic growth phase), were treated with 2.5, 5, and 10 ppm of AgNPs (US Research Nanomaterial Inc, Houston, TX, USA) and were harvested after 1 week (Rezaei et al. 2011).
Cell viability and biochemical analysis
Cell viability was determined by Evans blue assay. In brief, the cells to be assayed were incubated on a slide containing 0.05 % Evans blue for 5 min and then washed extensively with distilled water to remove excessive and unbound dye. The cells were observed and photographed by a light microscope (BH2, Olympus, Tokyo, Japan), equipped with a digital camera (Rezaei et al. 2011).
Determination of antioxidant enzymes activities was conducted using routine methods with some modifications (Ghanati et al. 2005). For determination of superoxide dismutase (SOD) activity the cells (0.2 g) were homogenized in 3 mL of HEPES–KOH buffer (50 mM, pH 7.8) containing 0.1 mM EDTA. The homogenate was centrifuged at 15,000×g for 15 min. The reaction mixture consisted of extraction buffer, 0.1 mM EDTA, 50 mM Na2CO3 (pH 10.2), 12 mM l-methionine, 75 µM nitroblue tetrazolium chloride (NBT), 300 µL enzyme extract, and 1 µM riboflavin and appropriate amount of the extracted enzyme. One unit of SOD activity was defined as the amount of enzyme that results in 50 % inhibition of the rate of NBT reduction at 560 nm and was expressed against mg of protein of the extract. Protein content was measured by Bradford method using bovine serum albumin (BSA) as a standard (Bradford 1976).
For measurement of catalase (CAT) activity the cells (0.2 g) were ground in 3 mL of sodium phosphate buffer (25 mM, pH 6.8), followed by centrifugation at 12,000×g for 20 min at 4 °C. Reaction mixture was composed of 10 mM H2O2 and diluted enzyme extract in a total volume of 3 mL. CAT activity was monitored by measuring decrease of absorbance at 240 nm due to H2O2 consumption, against milligram of protein.
Activity of peroxidase (POD) was measured in a reaction mixture which consisted of 60 mM Na-phosphate buffer (pH 6.1), 28 mM guaiacol, 5 mM H2O2 and enzyme extract. The increase in the absorbance was recorded at 470 nm and expressed against mg of protein.
For measurement of ascorbate peroxidase (APX) activity, the cells (0.2 g) were extracted in phosphate buffer 50 mM (pH 7.8) containing 5 mM DTT, 5 mM EDTA, 100 mM NaCl and 1 % (w/v) polyvinyl pyrrolidone. The reaction mixture was composed of aforesaid buffer in addition to 5 mM ascorbate, 44 µM H2O2, and appropriate amounts of the extract. The activity of APX was monitored by the decrease in absorbance at 290 nm. The rate constant was calculated using the extinction coefficient of 2.8 mM−1 cm−1 and corrected for the rate obtained prior to the addition of H2O2.
For determination of hydrogen peroxide content (H2O2), 0.2 g fresh cells were homogenized with 3 mL of 0.1 % (w/v) trichloracetic acid in an ice bath. The homogenate was centrifuged at 12,000×g for 10 min, then 0.5 mL of supernatant was mixed with 0.5 mL of 10 mM potassium phosphate buffer (pH 7) and 1 mL of 1 M KI. Absorbance of the mixture solution was read at 390 nm by a double beam spectrophotometer (Cintra 6, GBC, Dandenong, Vic, Australia). The content of H2O2 was determined based on a standard curve from 0 to 100 nM, and was expressed against fresh weight of sample (Rezaei et al. 2011).
The activity of phenylalanine ammonia-lyase (PAL) was determined based on the rate of cinnamic acid production. Briefly, aliquot of fresh cells (0.2 g) was homogenized in 3 mL of 50 mM Tris–HCl buffer containing 15 mM of β-mercaptoethanol (pH 8.2), in an ice-cooled mortar. The homogenate was centrifuged at 15,000×g for 20 min, and the supernatant was collected for enzyme assay. To 1 mL of the extraction buffer, 0.5 mL of 10 mM l-phenylalanine, 0.4 mL of double distilled water and 0.1 mL of enzyme extract were added and incubated at 37 °C for 1 h. The reaction was terminated by adding 0.1 mL of 6 M HCl, and the product (cinnamic acid) was extracted three times with 15 mL ethyl acetate, followed by evaporation to remove the extracting solvent. The solid residue was suspended in 3 mL of 0.05 M NaOH and its absorbance was measured at 290 nm. The cinnamic acid concentration wherein was quantified using a standard curve of 0–200 µM. One unit of PAL activity was considered equal to 1 µmol of cinnamic acid produced per min per mg of protein (Wang et al. 2006).
Total phenolic contents were determined using Folin–Ciocalteu reagent. In brief, 0.2 g fresh cells were homogenized in 3 mL methanol followed by centrifugation at 12,000×g for 8 min. To 0.5 mL of supernatant 0.5 mL of 1 N Folin–Ciocalteu reagent was added and the mixture was left for 2–5 min at ambient temperature. Then 1 mL of 20 % Na2CO3 was added and after 10 min incubation at ambient temperature, the absorbance was measured at 730 nm. Total phenolics was determined based on a standard curve of gallic acid and was expressed against fresh weight (Bemani et al. 2013).
Mesurement of Taxol content
For extraction of Taxol, the fresh cells were homogenized in 10 mL methanol followed by centrifugation at 5,000×g for 10 min. A mixture of methylene chloride and water (1:1) was added to the supernatant, mixed, and centrifuged again. Methylene chloride phase was collected, air-dried, re-dissolved in 150 µL of methanol, and filtered with a 0.45 µm syringe filter before applying for HPLC analysis. The HPLC system (Knauer, Berlin, Germany) was equipped with a C-18 column (Perfectsil Target ODS3, 4.6 × 250 mm, MZ-Analysentechnik, Mainz, Germany). Taxol was detected at 227 nm using an ultraviolet detector (PDA, Berlin, Germany). Quantification of Taxol was accomplished by comparison of retention time and peak area with genuine standard (Sigma, St. Louis, MO, USA) (Rezaei et al. 2011).
Statistical analysis
All experiments and observations were repeated three times each with at least three samples. One way ANOVA from SPSS (version 16, Chicago, IL, USA) was used, and a least significant difference (LSD) test was calculated for multiple means comparisons at a significance level of p ≤ 0.05.
Results
Viability and redox status of the cells
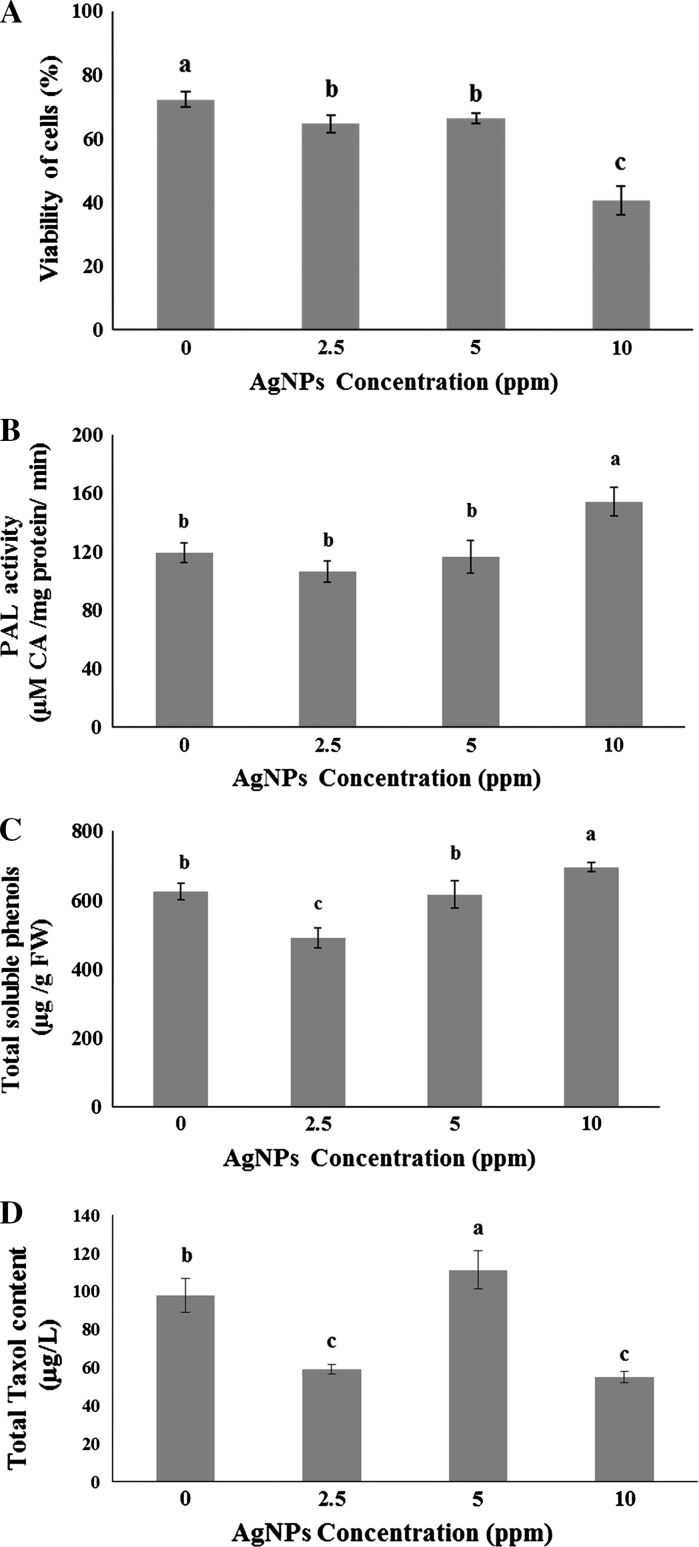
Treatment with AgNPs adversely affected the viability of hazel cells (Fig. 1a). The lowest viability (56 % of the control) was observed at the highest applied concentration of AgNPs (Fig. 1a).
Fig. 1.
a Viability of hazel cells before and after treatment with different concentrations of AgNPs. Data are mean ± SD, n = 3. Bars with different letters are significantly different at p ≤ 0.05 according to LSD test. b Activity of PAL in suspension-cultured hazel cells treated with different concentrations of AgNPs. Data are mean ± SD, n = 3. Different letters indicate significant differences at p ≤ 0.05 according to LSD. c The content of soluble phenolic compounds of hazel cells treated with or without different concentrations of AgNPs. Data are mean ± SD, n = 3. Different letters indicate significant differences at p ≤ 0.05 according to LSD. d Taxol production by suspension-cultured hazel cells treated with different concentrations of AgNPs. Data are mean ± SD, n = 3. Bars with different letters are significantly different at p ≤ 0.05 according to LSD test
The activity of SOD was significantly lowered by treatment with 2.5 and 5 ppm of AgNPs while the activity of CAT significantly increased in these treatments, compared to the control cells (Table 1).
Table 1.
The activities of CAT (ΔAbs 240/mg protein), POD (ΔAbs 470/mg protein), SOD (Unit/mg protein), APX (ΔAbs290/mg protein), and content of H2O2 (nmol/g FW) of suspension-cultured hazel cells before and after treatment with different concentrations of AgNPs
| AgNPs (ppm) | SOD | CAT | POD | APX | H2O2 |
|---|---|---|---|---|---|
| 0 | 130 ± 3a | 153 ± 17a | 925 ± 95b | 0.21 ± 0.0b | 30 ± 3.6c |
| 2.5 | 111 ± 2b | 194 ± 29b | 1,459 ± 273a | 0.27 ± 0.05ab | 28 ± 0.3c |
| 5 | 107 ± 5b | 166 ± 27b | 1,507 ± 193a | 0.14 ± 0.02c | 41 ± 2.6b |
| 10 | 128 ± 1a | 146 ± 4a | 1,088 ± 122a | 0.34 ± 0.05a | 48 ± 1.6a |
Data are mean ± SD, n = 3. Different letters indicate significant differences at p ≤ 0.05 according to LSD
The activity of POD in treatments containing 2.5, 5, and 10 ppm of AgNPs however, was significantly higher than in the control (158, 163, and 118 %, respectively) (Table 1). The maximum activity of APX and the highest content of H2O2 were observed in the cells treated with 10 ppm of AgNPs (Table 1). The highest activity of PAL was observed in those cells which were treated with 10 ppm of AgNPs (Fig. 1b). In comparison with control cells, treatment with 2.5 ppm AgNPs decreased total soluble phenolics content of hazel cells, while 5 ppm did not significantly change this parameter. The highest content of soluble phenolics was also observed in 10 ppm AgNPs-treated cells (Fig. 1c).
Taxol content
Treatment with 5 ppm AgNPs significantly increased the production of Taxol by hazel cells, compared to the control ones (Fig. 1d). Treatments containing 2.5 and 10 ppm AgNPs however, reduced the Taxol production potential of the cells to 60 and 56 % of the control, respectively (Fig. 1d).
Discussion
Nanoparticles are found to be reactive because of their small sizes leading to a high volume surface ratio which in turn leads to a lot of binding sites for metals and other compounds. This allows them to quite easily enter membrane and accumulate inside the cell and cause damages. Release of Ag+ from AgNPs has been shown by indirect evidences and a part of toxicity of AgNPs has been attributed to the production of higher levels of Ag+ (Navarro et al. 2008). The toxicity Ag+ is hypothesized to be due to disruption of membrane transport processes that disturb osmoregulation and finally lead to cell death (Dewez and Oukarroum 2012). Although the amount of intracellular Ag+ in hazel cells upon application of AgNPs, was not measured in the present study, this possibility should not be overlooked. Attachment of AgNPs itself to the cell membranes disturbs permeability and respiration and results in production of ROS (Massarsky et al. 2013).
Antioxidant enzymes are of the main ROS scavenging systems in plant cells, nonetheless some researchers have shown that the level of oxidative stress increase cannot entirely be attributed to a decrease in the activities of antioxidant enzymes and probably various factors may contribute to this process (Kasapoglu and Ozben 2001). The activity of SOD of hazel cells decreased by exposure to AgNPs. Interaction of AgNPs with copper dependent enzymes (e.g. SOD) and promotion of their denaturation has been suggested by Johnston et al. (2010). Biochemical analysis has also shown that the activity of Cu–Zn SOD significantly decreases following the application of AgNPs (Armstrong et al. 2013).
Although ROS scavenger enzymes act in a cooperative or synergistic way for the survival of the cell even under normal conditions, it is more likely that among them, CAT has the most critical role. This enzyme effectively eliminates H2O2 and has a very fast turnover rate, but a much lower affinity for H2O2 than APX and POD. Catalase scavenges the bulk of H2O2, thereby regulates the activity of other H2O2 scavengers i.e., APX and POD (Dat et al. 2000). Increase of both CAT and POD in a synergistic manner in 2.5 ppm AgNPs treatments resulted in sufficient scavenging of H2O2. In the presence of 5 ppm AgNPs, however, although CAT activity was higher than that of the control cells, the rate of its increase was not as high as 2.5 ppm AgNPs treatment. In addition to insufficient increase of CAT, reduction of APX activity resulted in failure of hazel cells to detoxify H2O2. The activity of POD increased in 2.5, 5, and 10 ppm AgNPs treatments. Comparison of the rate of the increase of POD, CAT, and APX activities and the amount of H2O2 in these treatments implies the hypothesis that the activity of POD is downstream of APX and the latter is regulated by CAT (Ghanati et al. 2005; Sahebjamei et al. 2007).
The content of H2O2 can be speculated as a central point which determines further direction of the cell fate, toward death or defense by producing secondary metabolites (Kumar et al. 2012). Phenolic compounds are of major secondary metabolite categories whose biosynthesis starts from the activity of the key enzyme PAL. It has been argued that H2O2 alone is not effective on PAL activity and cell viability, when it is generated at low concentration, whereas, when its production exceeds a threshold value, it induces cell death in a dose-dependent way (De Pinto et al. 2002). Investigating on the mechanism of birch response to different stresses, Pellinen et al. (2002) showed that H2O2 activated cell death and the expression of certain defense genes including PAL. Mejía-Teniente et al. (2013) also showed significant increase of PAL activity in Capsicum annum after application of H2O2 as an elicitor. They suggested that H2O2 acted as signal for expression of PAL gene thereby increased its enzyme activity. In the present study the lowest viability of hazel cells was observed in 10 ppm AgNPs treatment and was accompanied by the highest activity of PAL and the highest amounts of phenolic compounds. Although the real mechanism is not clearly understood, there is a possibility that increase of PAL activity in these cells, at least in part, is resulted from increase of their H2O2 content. It is likely that under lower concentrations of AgNPs hazel cell metabolism shifted toward production of Taxol. This fact was also evidenced by exogenous application of H2O2 (0–50 nM) in hazel cells medium in preliminary studies which showed a positive relationship between the concentration of H2O2 and the production of Taxol (y = 0.2485x − 0.2193, R2 = 0.9974, data not shown). The results presented here emphasizes once again on key role of H2O2 in determination of plant cell response to elicitors. Moreover, with regard to the importance of Taxol in cancer therapy and the necessity for elevation of its production by plant cell sources, treatment of hazel cells with 5 ppm AgNPs can be considered for further biotechnological experiments.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgments
This work was supported by grant no. 90000972 from the Iran National Science Foundation (INSF) to FGH on “The effects of MeJA and SNPs on medicinal compounds of plants”.
Abbreviations
- AgNPs
Silver nano particles
- APX
Ascorbate peroxidase
- CAT
Catalase
- H2O2
Hydrogen peroxide
- NBT
Nitroblue tetrazolium
- PAL
Phenylalanine ammonia lyase
- POD
Peroxidase
- ROS
Reactive oxygen species
- SOD
Superoxide dismutase
References
- Ahmed M, Posgai R, Gorey TJ, Nielsen M, Hussain SM, Rowe JJ. Silver nanoparticles induced heat shock protein 70, oxidative stress and apoptosis in Drosophila melanogaster. Toxicol Appl Pharmacol. 2010;242:263–269. doi: 10.1016/j.taap.2009.10.016. [DOI] [PubMed] [Google Scholar]
- An J, Zhang M, Wang S, Tang J. Physical, chemical and microbiological changes in stored green asparagus spears as affected by coating of silver nanoparticles—PVP. LWT Food Sci Technol. 2008;41:1100–1107. doi: 10.1016/j.lwt.2007.06.019. [DOI] [Google Scholar]
- Armstrong N, Ramamoorthy M, Lyon D, Jones K, Duttaroy A (2013) Mechanism of silver nanoparticles action on insect pigmentation reveals intervention of copper homeostasis. PLoS One 8:e53186. doi:10.1371/journal.pone.0053186 [DOI] [PMC free article] [PubMed]
- Bemani E, Ghanati F, Rezaei A, Jamshidi M. Effect of phenylalanine on Taxol production and antioxidant activity of extracts of suspension-cultured hazel (Corylus avellana L.) cells. J Nat Med. 2013;67:446–451. doi: 10.1007/s11418-012-0696-1. [DOI] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Dat J, Vandenabeele S, Vranová E, Van Montagu M, Inzé D, Van Breusegem F. Dual action of the active oxygen species during plant stress responses. Cell Mol Life Sci. 2000;57:779–795. doi: 10.1007/s000180050041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Pinto M, Tommasi F, De Gara L. Changes in the antioxidant systems as part of the signaling pathway responsible for the programmed cell death activated by nitric oxide and reactive oxygen species in tobacco bright-yellow 2 cells. Plant Physiol. 2002;130:698–708. doi: 10.1104/pp.005629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewez D, Oukarroum A. Silver nanoparticles toxicity effect on photosystem II photochemistry of the green alga Chlamydomonas reinhardtii treated in light and dark conditions. Toxicol Environ Chem. 2012;94:1536–1546. doi: 10.1080/02772248.2012.712124. [DOI] [Google Scholar]
- Ghanati F, Bakhtiarian S. Changes of natural compounds of Artemisia annua L. by methyl jasmonate and silver nanoparticles. Adv Environ Biol. 2013;7:2251–2258. [Google Scholar]
- Ghanati F, Morita A, Yokota H. Effects of aluminum on the growth of tea plant and activation of antioxidant system. Plant Soil. 2005;276:133–141. doi: 10.1007/s11104-005-3697-y. [DOI] [Google Scholar]
- Johnston HJ, Hutchison G, Christensen FM, Peters S, Hankin S, Stone V. A review of the in vivo and in vitro toxicity of silver and gold particulates: particle attributes and biological mechanisms responsible for the observed toxicity. Crit Rev Toxicol. 2010;40:328–346. doi: 10.3109/10408440903453074. [DOI] [PubMed] [Google Scholar]
- Kasapoglu M, Ozben T. Alterations of antioxidant enzymes and oxidative stress markers in aging. Exp Gerontol. 2001;36:209–220. doi: 10.1016/S0531-5565(00)00198-4. [DOI] [PubMed] [Google Scholar]
- Kim S, Choi JE, Choi J, Chung KH, Park K, Yi J, Ryu DY. Oxidative stress-dependent toxicity of silver nanoparticles in human hepatoma cells. Toxicol In Vitro. 2009;23:1076–1084. doi: 10.1016/j.tiv.2009.06.001. [DOI] [PubMed] [Google Scholar]
- Kumar RR, Sharma SK, Gadpayle KA, Singh K, Sivaranjani R, Goswami S, Rai RD. Mechanism of action of hydrogen peroxide in wheat thermotolerance-interaction between antioxidant isoenzymes, proline, and cell membrane. Afr J Biotechnol. 2012;11:14368–14379. doi: 10.5897/AJB12.1024. [DOI] [Google Scholar]
- Lü P, He S, Li H, Cao J, Xu H. Effects of nano-silver treatment on vase life of cut rose cv. Movie Star flowers. J Food Agric Environ. 2010;8:1118–1122. [Google Scholar]
- Massarsky A, Dupuis L, Taylor J, Eisa-Beygi S, Strek L, Trudeau VL, Moon TW. Assessment of nanosilver toxicity during zebrafish (Danio rerio) development. Chemosphere. 2013;92:59–66. doi: 10.1016/j.chemosphere.2013.02.060. [DOI] [PubMed] [Google Scholar]
- Mejía-Teniente L, de Dalia Duran-Flores F, Chapa-Oliver AM, Torres-Pacheco I, Cruz-Hernández A, González-Chavira MM, Ocampo-Velázquez RV, Guevara-González RG. Oxidative and molecular responses in Capsicum annuum L. after hydrogen peroxide, salicylic acid and chitosan foliar applications. Int J Mol Sci. 2013;14:10178–10196. doi: 10.3390/ijms140510178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarro E, Piccapietra F, Wagner B, Marconi F, Kaegi R, Odzak N, Sigg L, Behra R. Toxicity of silver nanoparticles to Chlamydomonas reinhardtii. Environ Sci Technol. 2008;42:8959–8964. doi: 10.1021/es801785m. [DOI] [PubMed] [Google Scholar]
- Nel A, Xia T, Madler L, Li N. Toxic potential of materials at the nanolevel. Science. 2006;311:622–627. doi: 10.1126/science.1114397. [DOI] [PubMed] [Google Scholar]
- Pellinen RI, Korhonen MS, Tauriainen AA, Palva ET, Kangasjärvi J. Hydrogen peroxide activates cell death and defense gene expression in Birch. Plant Physiol. 2002;130:549–560. doi: 10.1104/pp.003954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rezaei A, Ghanati F, Behmanesh M, Mokhtari Dizaji M. Ultrasound-potentiated salicylic acid-induced physiological effects and production of Taxol in hazel (Corylus avellana L.) cell culture. Ultrasound Med Biol. 2011;37:1938–1947. doi: 10.1016/j.ultrasmedbio.2011.06.013. [DOI] [PubMed] [Google Scholar]
- Sahebjamei H, Abdolmaleki P, Ghanati F. Effects of magnetic field on the antioxidant enzyme activities of suspension-cultured tobacco cells. Bioelectromagnetics. 2007;28:42–47. doi: 10.1002/bem.20262. [DOI] [PubMed] [Google Scholar]
- Sondi I, Salopek-Sondi B. Silver nanoparticles as antimicrobial agent: a case study on E. coli as a model for Gram-negative bacteria. J Colloid Interface Sci. 2004;285:148–177. doi: 10.1016/j.jcis.2004.02.012. [DOI] [PubMed] [Google Scholar]
- Wang JW, Zheng LP, Wu JY, Tan RX. Involvement of nitric oxide in oxidative burst, phenylalanine ammonia-lyase activation and Taxol production induced by low-energy ultrasound in Taxus yunnanensis cell suspension cultures. Nitric Oxide. 2006;15:351–358. doi: 10.1016/j.niox.2006.04.261. [DOI] [PubMed] [Google Scholar]
- Wu Y, Zhou Q. Silver nanoparticles cause oxidative damage and histological changes in medaka (Oryzias latipes) after 14 days of exposure. Environ Toxicol Chem. 2013;32:165–173. doi: 10.1002/etc.2038. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.