Abstract
Despite the well established ivermectin activity against microfilaria, the success of human filariasis control programmes requires the use of a macrofilaricide compound. Different in vivo trials suggest that flubendazole (FLBZ), an anthelmintic benzimidazole compound, is a highly efficacious and potent macrofilaricide. However, since serious injection site reactions were reported in humans after the subcutaneous FLBZ administration, the search for alternative pharmaceutical strategies to improve the systemic availability of FLBZ has acquired special relevance both in human and veterinary medicine. The goal of the current experimental work was to compare the pharmacokinetic plasma behavior of FLBZ, and its metabolites, formulated as either an aqueous hydroxypropyl- β -cyclodextrin-solution (HPBCD), an aqueous carboxymethyl cellulose-suspension (CMC) or a Tween 80-based formulation, in pigs. Animals were allocated into three groups and treated (2 mg/kg) with FLBZ formulated as either a HPBCD-solution (oral), CMC-suspension (oral) or Tween 80-based formulation (subcutaneous). Only trace amounts of FLBZ parent drug and its reduced metabolite were measured after administration of the different FLBZ formulations in pigs. The hydrolyzed FLBZ (H-FLBZ) metabolite was the main analyte recovered in the bloodstream in pigs treated with the three experimental FLBZ formulations. The oral administration of the HPBCD-solution accounted for significantly higher (P < 0.05) Cmax and AUC (23.1 ± 4.4 μg h/mL) values for the main metabolite (H-FLBZ), compared with those observed for the oral CMC-suspension (AUC = 3.5 ± 1.0 μg h/mL) and injectable Tween 80-based formulation (AUC: 7.5 ± 1.7 μg h/mL). The oral administration of the HPBCD-solution significantly improved the poor absorption pattern (indirectly assessed as the H-FLBZ plasma concentrations) observed after the oral administration of the FLBZ-CMC suspension or the subcutaneous injection of the Tween 80 FLBZ formulation to pigs. Overall, the work reported here indicates that FLBZ pharmacokinetic behavior can be markedly changed by the pharmaceutical formulation.
Keywords: Flubendazole, Systemic exposure, Pharmaceutical preparations, Cyclodextrins
Graphical abstract
Highlights
-
•
The pharmacokinetics of three different FLBZ formulations was assessed in pigs.
-
•
Hydrolyzed-FLBZ was the main metabolite detected in pigs given the formulations.
-
•
Traces of FLBZ and reduced-FLBZ were measured after administration of FLBZ.
-
•
Oral administration of FLBZ-HPBCD solution resulted in a high systemic H-FLBZ exposure.
-
•
Similar FLBZ plasma exposure was observed after parenteral FLBZ-Tween 80 and HPBCD administration.
1. Introduction
The control and eventual elimination of neglected tropical diseases (NTD) requires the expansion of interventions such as mass drug administration (MDA), vector control, diagnostic testing and effective treatment. Current efforts in this area of tropical public health have been aimed at onchocerciasis and lymphatic filariasis (LF) as well as loiasis as co-infection with this other filaria can cause severe adverse reaction in MDA areas. These diseases continue to cause widespread sickness and disability in many parts of the world. Of the total population requiring preventive chemotherapy for LF, 57% live in the South-East Asia Region and 38% live in the African Region. Onchocerciasis is endemic in 31 countries in Africa and 6 countries in Latin America, with 99% of cases of onchocerciasis-related blindness found in Africa (WHO, 2013).
Chemotherapy remains the main approach to treatment, control, and elimination of filarial infections aided where suitable by adjunct activities such as vector control and enhanced program management. Current onchocerciasis therapy employ ivermectin (IVM) against microfilariae (mf) in the skin and against further production of mf by female worms (Basáñez et al., 2008). These effects reduce the pathology of the disease and prevent transmission by blocking repopulation of the skin with mf for >6 months following a single dose. However, due to the long life-span of the adult parasites, eradication of the disease will almost certainly require a field-compatible macrofilaricide. Existing macrofilaricides have either unacceptable toxicity (e.g., suramin) (Awadzi, 2003) or induce severe adverse reactions contraindicating their use at doses required to be effective (e.g., diethylcarbamazine (DEC) (Bird et al., 1979). For LF, DEC or IVM in combination with albendazole (ABZ) are used as the basis for the global programme for elimination, but the availability of a single-dose macrofilaricide would provide chemotherapeutic options that could significantly reduce program duration (Taylor et al., 2010).
Although IVM has had an enormous impact on onchocerciasis and LF, this agent lacks the ability to kill the adult parasites. Since the adult worms can survive for many years, it has been necessary for control programs to continue drug distribution for more than a decade, for instance, until the adult worms eventually die (Mackenzie and Geary, 2011). As a consequence, the search for a macrofilaricide that can enhance elimination of filarial infections, and the diseases they cause, is a current and relevant goal. Different in vivo trials suggest that flubendazole (FLBZ), a benzimidazole methylcarbamate anthelmintic currently licensed for use in humans for treatment of infection by intestinal nematodes (EMEA, 1997), is a highly efficacious and potent macrofilaricide in experimental animals when given parenterally (Zahner and Schares, 1993, Mackenzie and Geary, 2011), including in the feline Brugia pahangi model, a host in which this parasite occurs naturally. In addition, FLBZ has been reported to be able to eliminate adult Dirofilaria immitis from dogs after a single injection (Mackenzie and Geary, 2011). It is important to stress that FLBZ is macrofilaricidal in these models only when given parenterally (as a consequence of its very low oral bioavailability in standard formulations). Studies in humans infected with Onchocerca volvulus reported problems associated with reactions at the intramuscular injection site where FLBZ, in its oil-based carrier, was administered.
As a chemical class, the benzimidazole methylcarbamates have very limited water solubility, which allows their preparation only as tablets/suspensions for oral administration in humans. Small differences in drug solubility may have a major influence on their absorption and resultant pharmacokinetic behavior (Lanusse et al., 1995). It has been reported that the use of complexing agents such as hydroxypropyl-β-cyclodextrins (HPBCD) increases the water solubility of FLBZ and ABZ (Ceballos et al., 2012) and their systemic drug exposure in different species (Evrard et al., 2002, Ceballos et al., 2009, Ceballos et al., 2014). Similar findings have been reported in humans (Rigter et al., 2004).
Prospects for an accelerated path to the elimination of onchocerciasis and LF would be much enhanced if a safe and effective macrofilaricide was available (Geary et al., 2010, Mackenzie and Geary, 2011). Bioavailability is a key pharmacokinetic parameter, defined as the proportion of a drug administered by a nonvascular route that gains access to the systemic circulation (Toutain and Bousquet-Melou, 2004). Bioavailability quantifies the proportion of a drug which is available to produce systemic effects. When pharmacological research cannot be done on humans for practical and ethical reasons, animal models constitute an acceptable alternative approach to understand the parasite-drug-host relationship.
In this context, the search for alternative pharmaceutical strategies to improve FLBZ oral bioavailability may be considered critical to optimize its pharmacological activity. The goal of the current experimental work was to compare the pharmacokinetic behavior of FLBZ, and its metabolites, formulated as either an aqueous hydroxypropyl- β -cyclodextrin-solution, an aqueous carboxymethyl cellulose-suspension or a Tween 80-based formulation, in pigs.
2. Materials and methods
2.1. Chemicals
Pure reference standards of FLBZ, reduced-FLBZ (R-FLBZ) and hydrolyzed-FLBZ (H-FLBZ) used to develop the analytical methodology were kindly provided by Janssen Animal Health (Beerse, Belgium). Oxibendazole (OBZ), used as internal standard, was obtained from Schering Plough (Kenilworth, NJ, USA). HPLC grade acetonitrile and methanol were from Sintorgan S.A. (Buenos Aires, Argentina) and J.T. Baker (Phillipsburg, New Jersey, USA), respectively. HPBCDs were from ISP Pharmaceuticals (Cavasol, New Jersey, USA). Low viscosity grade sodium CMC was purchased from Anedra (Buenos Aires, Argentina). Tween® 80 was purchased from Biopack (Buenos Aires, Argentina).
2.2. Preparation of FLBZ formulations
The FLBZ HPBCD-based solution was prepared by dissolving FLBZ (0.1%) and HPBCD (10%) in deionized water. The pH of the formulation was adjusted to 1.2 using HCl (25 mM). The formulation was shaken until total dissolution of the drug and then was filtrated through a 0.45 μm filter (Whatman, NJ, USA). The final FLBZ concentration was confirmed by HPLC. The Tween 80-based formulation was prepared by dissolving FLBZ (0.25%) in Tween 80. The FLBZ-suspension was prepared by addition of FLBZ (0.1%) and CMC (0.1%) in deionized water (pH = 6.0) with shaking for 6 h. The FLBZ-CMC suspension was vigorously shaken immediately before administration to pigs. FLBZ formulations were freshly prepared and maintained under refrigeration (3–5 °C).
2.3. Experimental animals
Sixteen (16) healthy pigs (45.2 ± 7.54 kg) were used in two different experiments. Pigs were fed ad libitum with a commercial balanced food and had free access to water. Animals were housed in pens with concrete floors, protected from rain and prevailing winds, but without temperature control. Animal procedures and management protocols were approved by the Ethics Committee according to the Animal Welfare Policy (act 087/02) of the Faculty of Veterinary Medicine, Universidad Nacional del Centro de la Provincia de Buenos Aires (UNCPBA), Tandil, Argentina (http://www.vet.unicen.edu.ar).
2.4. Experimental design
Two different experiments were undertaken to generate the data reported in this article. Experiment 1 was a crossover study involving two experimental phases. In phase I, pigs received the following treatments: FLBZ-HPBCD solution (Cavasol®, n = 4, oral treatment) and FLBZ-CMC suspension (n = 4, oral treatment). After a 21-day washout period, drug treatments were reversed for each drug (phase II). Additionally in experiment 2, eight pigs were dosed subcutaneously with the FLBZ-Tween 80 solution. All treatments were given as a single dose of 2 mg/kg. Blood samples (5 mL) were taken from all pigs by anterior vena cava venipuncture into heparinized Vacutainers® tubes (Becton Dickinson, Franklin Lakes, NJ, USA), prior to treatment and at 1, 2, 3, 6, 9, 12, 15, 24, 30, 34, 48 and 54 h post-treatment for oral treated groups (experiment 1) and up to 72 h post-treatment for subcutaneous administration (experiment 2). Plasma was separated by centrifugation at 2000 × g for 15 min. Plasma samples were placed into plastic vials and stored at −20 °C until analyzed by HPLC.
2.5. Analysis of FLBZ and its metabolites
Chromatography was performed on a Shimadzu HPLC platform (Shimadzu Corporation, Kyoto, Japan), with two LC-10AS solvent pumps, an automatic sample injector (SIL-10A) with a 50 μL loop, an ultraviolet–visible spectrophotometric detector (UV) (SPD-10A) reading at 292 nm, a column oven (Eppendorf TC-45, Eppendorf, Madison, WI, USA) set at 30 °C, and a CBM-10A integrator. Data and chromatograms were collected and analyzed using the Class LC10 software (SPD-10A, Shimadzu Corporation, Kyoto, Japan). The C18 reversed-phase column (5 μm, 250 mm × 4.6 mm) was Kromasil (Kromasil®, Sweden). Elution from the stationary phase was carried out at a flow rate of 1.2 mL/min using an acetonitrile (34%)/ammonium acetate buffer (0.025 M, pH 5.3, 66%) as a mobile phase.
Plasma samples (500 μL) were spiked with OBZ (20 μL) as internal standard. FLBZ and its metabolites were extracted using disposable cartridges (Strata®, Phenomenex, CA, USA) previously conditioned with 0.5 mL of methanol, followed by 0.5 mL of water, as described (Moreno et al., 2004). All samples were injected into cartridges and then sequentially washed with 1.5 mL of water and eluted with 2 mL of methanol. Identification of FLBZ and its metabolites was undertaken by comparison with retention times of pure reference standards. Complete validation of the analytical procedures for extraction and quantification of drug and metabolites in plasma was performed before starting the analysis of experimental samples. Retention times for H-FLBZ, R-FLBZ and FLBZ were 5.7, 7.1 and 14.4 min, respectively. Calibration curves for each analyte, constructed by least squares linear regression analysis, showed good linearity, with correlation coefficients ≥0.998. The limit of quantification (FLBZ and metabolites), defined as the lowest measured concentration with a CV <20%, accuracy of ±20% and absolute recovery ≥ 70%, was 0.01 μg/mL.
2.6. Pharmacokinetic analysis of the data
Non-compartmental pharmacokinetic analysis for the plasma concentration versus time curves for H-FLBZ for each individual animal after FLBZ treatment was conducted using PK Solution 2.0 (Summit research services, CO, USA). The low concentrations of FLBZ and R-FLBZ quantified during a short time period precluded the development of a complete pharmacokinetic analysis of the data obtained for these molecules after the oral or subcutaneous administration of FLBZ. The peak concentration (Cmax) and time to peak concentration (Tmax) for each individual animal were read from the plotted concentration–time curve for each analyte. The elimination half-life (T½el) and absorption (T½ab) or metabolite formation (T½for) half-lives were calculated as ln2/β and ln 2/k, respectively. The area under the concentration–time curve from 0 to the limit of quantification (AUC0-LOQ) for FLBZ and metabolites was calculated by the trapezoidal rule (Gibaldi and Perrier, 1982).
2.7. Statistical analysis
Pharmacokinetic parameters are reported as arithmetic mean ± SD. The mean pharmacokinetic parameters obtained for each experimental group in experiment 1 were compared by paired t-test. Additionally, pharmacokinetic parameters obtained from experimental groups involved in experiment 1 and 2 were compared by analysis of variance (ANOVA). A parametric (ANOVA + Tukey) and non-parametric (Mann–Whitney) were used where significantly differences among standard deviations were observed. Mean values were considered significantly different at P < 0.05. The statistical analysis was performed using Instat 3.0 Software (Graph Pad Software, CA, USA).
3. Results
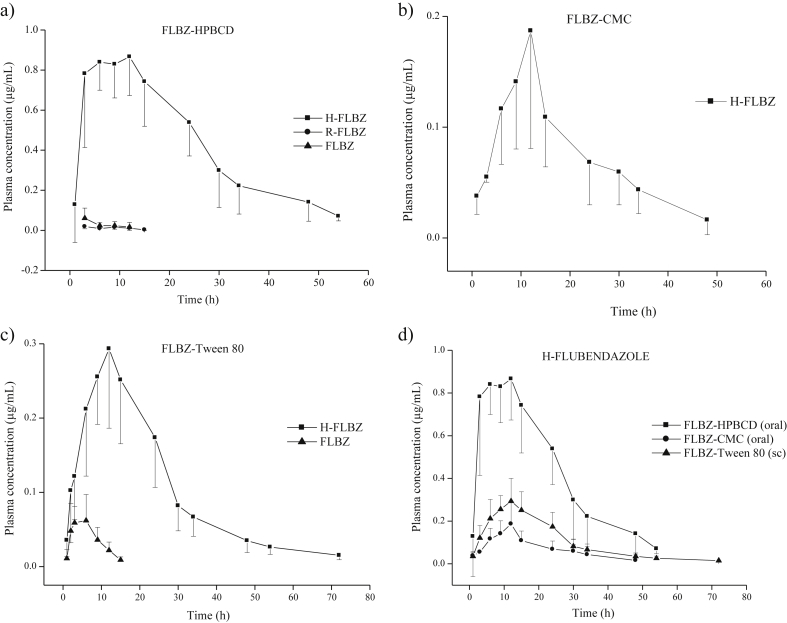
During treatment and follow up, pigs from treated groups were apparently normal without adverse events such as diminished food/water consumption, ataxia, etc. A local tissue reaction at the administration site was observed after subcutaneous administration of FLBZ formulated in Tween 80. This reaction was characterized by swelling, discoloration (which was more evident at 7 days post-injection) and slight alopecia. Fig. 1 shows the mean (±SD) plasma concentration profiles for FLBZ and its metabolites after oral or subcutaneous administration of the three FLBZ formulations.
Fig. 1.
a) b) and c) Plasma concentration profiles (mean ± SD) of flubendazole (FLBZ) and its metabolites hydrolyzed-FLBZ (H-FLBZ) and reduced-FLBZ (R-FLBZ) after administration of FLBZ (2 mg/kg) formulated as either a) hydroxypropyl-β-cyclodextrin (FLBZ-HPBCD)-based solution (oral), b) a conventional carboxymethyl-cellulose suspension (FLBZ-CMC) (oral) or c) a Tween 80-based formulation (FLBZ-Tween 80) (subcutaneous) to healthy pigs. FLBZ parent drug was not detected after administration of the suspension, d) Comparative plasma concentrations (mean ± SD) of the hydrolyzed-flubendazole (H-FLBZ) metabolite (main analyte recovered in the bloodstream) obtained after administration of the three different formulations.
The only FLBZ metabolite recovered from plasma samples after oral administration of the FLBZ-CMC suspension was H-FLBZ, which was quantified in plasma between 1 and 48 h post-treatment (Fig. 1b). For these reasons, comparison of relative availability among the different FLBZ formulations was based on the plasma levels of H-FLBZ, the main analyte found in the bloodstream of FLBZ-treated pigs. Fig. 1 d shows the comparative (mean ± SD) plasma concentration profiles of H-FLBZ obtained after oral administration of FLBZ formulated as an HPBCD-, a conventional CMC-suspension or a Tween 80-based formulation to healthy pigs. Table 1 summarizes the main pharmacokinetic parameters (mean ± SD) for H-FLBZ obtained after oral or subcutaneous administration of FLBZ as different formulations. H-FLBZ was detected at 1 h post-treatment (first sampling point), and rapidly increased to reach a Cmax of 1.01 ± 0.1 μg/mL (FLBZ-HPBCD), 0.2 ± 0.1 μg/mL (FLBZ-CMC) and 0.3 ± 0.1 μg/mL (FLBZ-Tween 80) at a Tmax ranging 9.7–10.5 h post-treatment (Table 1). Cmax values for H-FLBZ were significantly (P < 0.05) higher in pigs treated with the FLBZ-HPBCD formulation (Table 1) than with the CMC or Tween 80 formulations. Additionally, H-FLBZ systemic exposure (expressed as AUC0-LOQ) was significantly higher (P < 0.05) in the FLBZ-HPBCD group (23.1 ± 4.4 μg h/mL) compared to the oral CMC-suspension (3.5 ± 1.0 μg h/mL) or the injectable Tween 80-based formulation (7.5 ± 1.7 μg h/mL) groups. This represents an increment in the AUC0-LOQ value of 560% (compared to the CMC-based formulation) and 208% (compared to the Tween 80-based formulation) in the pigs treated with FLBZ-HPBCD. After parenteral (FLBZ-Tween 80) or oral (FLBZ-CMC) administration of FLBZ, a similar Cmax value for H-FLBZ was observed. However, since this metabolite was detected longer after the parenteral treatment, a higher (P < 0.05) AUC0-LOQ value was obtained in this experimental group compared to the group treated with the CMC-based suspension (Table 1). There were no major differences (P > 0.05) in other pharmacokinetic parameters obtained for H-FLBZ such as Tmax, T½for and T½el among formulations. The FLBZ-CMC formulation showed the lowest relative availability. Neither the parent drug nor the R-FLBZ metabolite could be quantified in any plasma sample belonging to this group, since all concentrations were below the limit of quantification (0.01 μg/mL).
Table 1.
Pharmacokinetic parameters (mean ± SD) for the hydrolyzed flubendazole (H-FLBZ) metabolite obtained after the oral administration of flubendazole (FLBZ, 2 mg/kg) formulated as either a hydroxypropyl-β-cyclodextrin solution (HPBCD), a carboxymethyl cellulosa-suspension (CMC), or a tween 80-based formulation (Tween 80) to healthy pigs.
| Pharmacokinetic parameters | H-Flubendazole |
||
|---|---|---|---|
| HPBCD | CMC | Tween 80 | |
| Cmax (μg/mL) | 1.01 ± 0.1a | 0.2 ± 0.1b∗ | 0.32 ± 0.1b |
| Tmax (h) | 9.7 ± 5.1a | 10.5 ± 1.7a | 10.5 ± 2.5a |
| AUC0-LOQ (μg.h/mL) | 23.1 ± 4.4a | 3.5 ± 1.0b∗ | 7.5 ± 1.7c |
| T½for (h) | 2.3 ± 1.3a | 4.9 ± 1.6a | 3.2 ± 1.2a |
| T½el (h) | 9.81 ± 1.9a | 13.2 ± 7.6a | 11.3 ± 2.3a |
Cmax: peak plasma concentration; Tmax: time to the Cmax; AUC0–LOQ: area under the plasma concentration vs. time curve from 0 to the limit of quantification; T½ for: metabolite formation half life; T½el: elimination half-life.* Indicates statistically significant differences (P<0.05) between FLBZ-HPBCD and FLBZ-CMC groups (paired t test). Different superscripts indicate statistically significant differences (P<0.05) among experimental groups.
Trace amounts of FLBZ and R-FLBZ were measured in plasma after oral administration of FLBZ as an HPBCD formulation reaching maximum concentrations of 0.06 ± 0.05 μg/mL and 0.04 ± 0.03 μg/mL for parent drug and active metabolite, respectively (Fig. 1a). Additionally, after subcutaneous administration as a Tween 80-based formulation, low FLBZ plasma concentrations were measured up to 15 h post-treatment with a Cmax value of 0.06 ± 0.03 μg/mL (Fig. 1c). It is important to highlight that the plasma concentrations for FLBZ and R-FLBZ (Fig. 1a and c) represent the mean values obtained from all experimental animals in each group. In both groups (HPBCD and Tween 80), most of the individual concentrations were below the LOQ. Consequently, the low number of “concentration points” available from each animal precluded any individual pharmacokinetic analysis. The basic pharmacokinetic data obtained for FLBZ are shown in Table 2.
Table 2.
Pharmacokinetic parameters (mean ± SD) for flubendazole (FLBZ) obtained after the oral administration of flubendazole (FLBZ, 2 mg/kg) formulated as either a hydroxypropyl-β-cyclodextrin solution (HPBCD) or a tween 80-based formulation (Tween 80) to healthy pigs.
| Pharmacokinetic parameters | Flubendazole |
|
|---|---|---|
| HPBCD | Tween 80 | |
| Cmax (μg/mL) | 0.06 ± 0.05 | 0.06 ± 0.03 |
| Tmax (h) | 6.00 ± 3.00 | 3.14 ± 1.35 |
| AUC0-LOQ (μg.h/mL) | 0.40 ± 0.26 | 0.39 ± 0.20 |
Cmax: peak plasma concentration; Tmax: time to the Cmax; AUC0–LOQ: area under the plasma concentration vs. time curve from 0 to the limit of quantification. FLBZ was not quantified after its administration as a carboxymethyl cellulosa-suspension. P > 0.05.
4. Discussion
Pigs, dogs, and monkeys are the most common non-rodent species used in toxicity testing of drugs (Dalgaard, 2015). The current report gives an overview of the metabolism and disposition of FLBZ/metabolites in treated pigs to approximate what might be expected in humans. FLBZ is a highly efficacious macrofilaricide in a variety of experimental animals, and is a potent and efficacious anthelmintic for gastrointestinal nematode infections in pigs, poultry and domestic animals (Mackenzie and Geary, 2011). Knowledge of anthelmintic drug concentrations (active molecules) achieved in tissues/fluids at the parasites location will contribute to the understanding of the pharmacokinetics-efficacy relationship. Plasma concentration profiles reflect those attained for the different fluid/tissues where target parasite may be located. Consequently, this characterization contributes to understanding the relationship between drug concentration and clinical efficacy, which is useful to optimize parasite control. Usually, the higher the concentration achieved in the tissue/fluid where the parasite is located, the higher the amount of drug reaching the parasite. This is strongly supported by the findings from in vivo studies (Hennessy et al., 1995, Alvarez et al., 2000, Lloberas et al., 2012).
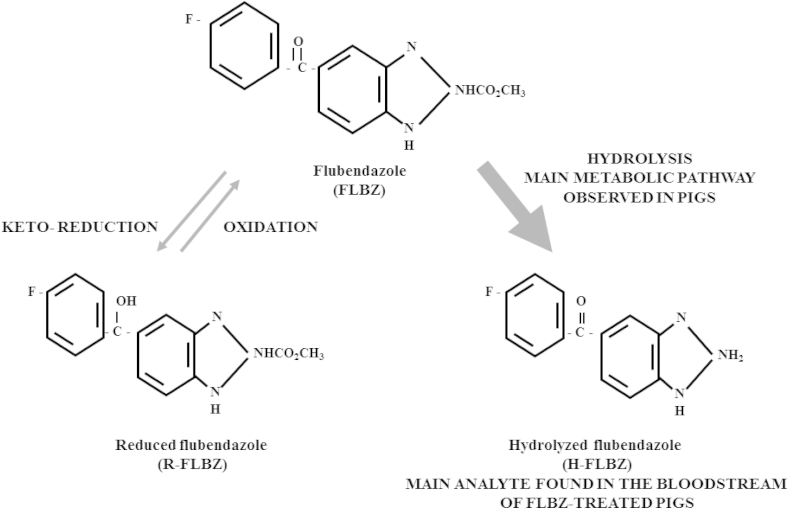
Lipophilic benzimidazole anthelmintics such as FLBZ require extensive hepatic oxidative metabolism to achieve sufficient polarity for excretion. Carbonyl reductases (CBRs) are primarily involved in FLBZ biotransformation. The main FLBZ metabolic pathways include reduction of the ketone group to form R-FLBZ, and hydrolysis of the methylcarbamate group to form H-FLBZ. The contribution of each metabolite to the total amount of drug recovered from plasma after FLBZ treatment may differ according to the animal species considered (Maté et al., 2008). Available data appear to indicate that, while H-FLBZ has no anthelmintic activity, R-FLBZ is an active metabolite. The in vitro protoscolicidal (Ceballos et al., 2011) and in vivo nematocidal (Urbizu et al., 2012) effects of R-FLBZ have been described. Additionally, its effect on Fasciola hepatica egg hatching was reported (Alvarez et al., 2009). While R-FLBZ is the main FLBZ metabolite found in plasma of sheep (Moreno et al., 2004) and mice (Ceballos et al., 2009), H-FLBZ is the metabolite which predominates in the systemic circulation of pigs after FLBZ treatment (Fig. 2), which may represent a disadvantage in this animal species in terms of the final anthelmintic activity of FLBZ against systemically located target parasites.
Fig. 2.
Main FLBZ metabolic pathways in pigs. These include reduction of the ketone group to form reduced flubendazole (R-FLBZ) and hydrolysis of the methylcarbamate group to form hydrolyzed flubendazole (H-FLBZ).
We found in the present work that the hydrolyzed metabolite was the main molecule recovered from pigs treated with FLBZ, representing 97% of the total drug detected in plasma (Fig. 2). Only trace of FLBZ parent drug and R-FLBZ could be quantified up to 12 h (FLBZ) and 15 h (R-FLBZ) post-treatment (HPBCD-based formulation). In agreement with these results, in vitro studies performed in our laboratory revealed that the rate of production of H-FLBZ by liver microsomal fractions obtained from pigs was 6.93-fold higher than R-FLBZ production (Maté et al. unpublished observations). Furthermore, a different plasma profile was observed in rats, since similar amounts of FLBZ and H-FLBZ were present in the bloodstream, with only trace amounts of R-FLBZ (Ceballos et al., 2014). Although oral bioavailability of FLBZ has been estimated in humans (EMEA, 1997), no data are available on the plasma pharmacokinetic pattern of FLBZ and metabolites. However, in vitro studies carried out in our laboratory have shown that human liver microsomes produced similar amounts of each metabolite, with the ratio H-FLBZ/R-FLBZ = 1.30 (Maté et al. unpublished observations). These results may indicate that the FLBZ metabolic profile in humans differs from that in pigs and is instead more similar to the metabolic profile observed in mice (Ceballos et al., 2014). The potential in vivo metabolism of FLBZ to R-FLBZ in humans may be important in terms of anthelmintic activity, since as previously mentioned, this metabolite is anthelmintically active.
Clearly, the poor oral absorption of FLBZ after administration in conventional suspension/tablet formulations is a serious disadvantage for the treatment of systemic infections such as filariases. Strategies to attain satisfactory oral bioavailability through new formulations of FLBZ should be evaluated. Enhanced aqueous solubility and bioavailability of guest molecules is a common effect observed after drug formulation with cyclodextrins (CDs) (Loftsson and Duchene, 2007). We have previously reported that incorporation of FLBZ into an HPBCD formulation significantly increased its water solubility (Ceballos et al., 2012) and systemic exposure in mice by more than 25-fold compared to the conventional FLBZ suspension (Ceballos et al., 2009). The relative bioavailability of albendazole sulphoxide (ABZSO) in mice was also increased by formulation with CDs (Garcia et al., 2003). Similar findings have been reported in humans (Rigter et al., 2004). Since the parent BZD compounds are not detected or are detected in very low concentrations in plasma, the relative availability of benzimidazole methylcarbamate anthelmintics are commonly assessed trough the analysis of the plasma concentration profiles of their metabolites (Evrard et al., 2002, Ceballos et al., 2009, Suarez et al., 2011). Thereby, increased parent drug absorption is related to higher metabolite plasma exposure. In the present work, the use of an HPBCD-based formulation of FLBZ orally administered to pigs induced drastic changes in the H-FLBZ plasma availability, resulting in significantly higher plasma Cmax and AUC values compared to those obtained after dosing with the FLBZ suspension or the FLBZ Tween 80-based formulation subcutaneously administered (Fig. 1d) (P < 0.05).
Local injection site adverse effects were observed in animals after subcutaneous treatment, which could be related to a reaction to the solvent used in the formulation (Tween 80), post-injection drug precipitation and/or the relatively high volume injected (30 mL approx.). Irritation and post-injection precipitation are concerns in parenteral drug delivery for poorly water soluble drugs (Muller et al., 2004). Post-injection drug precipitation may cause problems in several ways: (1) mechanical irritation caused by particles of the precipitated drug; (2) irritation to tissues at the injection site due to the prolonged drug-tissue contact time; and (3) the possibility of poor and less reproducible systemic bioavailability (Wu et al., 2010). That is in concordance with a low relative plasma availability achieved for H-FLBZ after Tween 80 subcutaneous administrations to pigs. Equivalent FLBZ plasma concentration profiles were observed after the HPBCD and the Tween 80 formulation. Since in pigs the main active molecule after FLBZ treatment is the parent drug (H-FLBZ is an inactive metabolite), a similar anthelmintic efficacy against systemic parasites could be expected after the use of HPBCD and the Tween 80 formulation. However, if in humans a portion of FLBZ can be reduced to R-FLBZ, a high macrofilaricidal efficacy could be expected after the HPBCD oral administration. This hypothesis needs to be explored.
Overall, the work reported here demonstrates that FLBZ pharmacokinetic behavior can be markedly altered by changes in formulation. The cyclodextrin-based formulation improved the absorption of FLBZ compared to the conventional CMC suspension, and reached similar FLBZ plasma concentrations than that observed after the subcutaneous administration of the Tween 80-based formulation. If an equivalent (similar) pharmacokinetic behavior can be achieved in humans, it is highly likely that a macrofilaricidal efficacy could be obtained after FLBZ oral administration as a HPBCD-based formulation. However, this formulation faces two main impediments: a) the high cost of the HPBCD excipient and b) the large volume required for potential treatment of humans. Thus, a crucial next step in the development of FLBZ as a macrofilaricide may be the reduction of costs associated with CD or alternatively, the selection of a low cost excipient contributing the same benefits as CDs, enabling an increase in the FLBZ concentration in the oral pharmaceutical preparation.
Acknowledgments
This work was partially supported by Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET), Agencia Nacional de Promoción Científica y Técnica (ANPCyT, both from Argentina) and Bill and Melinda Gates Foundation (through DNDi, Geneva). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. The authors acknowledge Dr. Kathleen Vlaminck, Dr. Leo Van Leemput (Janssen Animal Health, Beerse, Belgium) and Dr. Gustavo Viana (Janssen, Buenos Aires, Argentina) for providing the FLBZ and metabolites used in the current work.
References
- Alvarez L., Imperiale F., Sánchez S., Murno G., Lanusse C. Uptake of albendazole and albendazole sulphoxide by Haemonchus contortus and Fasciola hepatica in sheep. Vet. Parasitol. 2000;94:75–89. doi: 10.1016/s0304-4017(00)00320-4. [DOI] [PubMed] [Google Scholar]
- Alvarez L., Moreno G., Moreno L., Ceballos L., Shaw L., Fairweather I., Lanusse C. Comparative assessment of albendazole and triclabendazole ovicidal activity on Fasciola hepatica eggs. Vet. Parasitol. 2009;164:211–216. doi: 10.1016/j.vetpar.2009.05.014. [DOI] [PubMed] [Google Scholar]
- Awadzi K. Clinical picture and outcome of serious adverse events in the treatment of onchocerciasis. Filaria J. 2003;2(Suppl. 1):S6. doi: 10.1186/1475-2883-2-S1-S6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basáñez M., Pion S.D., Boakes E., Filipe J.A., Churcher T.S., Boussinesq M. Effect of single-dose ivermectin on Onchocerca volvulus: a systematic review and meta-analysis. Lancet Infect. Dis. 2008;8(5):310–322. doi: 10.1016/S1473-3099(08)70099-9. [DOI] [PubMed] [Google Scholar]
- Bird A., El-Sheikh H., Anderson J., Fuglsang H. Visual loss during oral diethylcarbamazine treatment for onchocerciasis. Lancet. 1979;2:8132–8146. doi: 10.1016/s0140-6736(79)90214-9. [DOI] [PubMed] [Google Scholar]
- Ceballos L., Elissondo M., Bruni S., Denegri G., Alvarez L., Lanusse C. Flubendazole in cystic echinococcosis therapy: pharmaco-parasitological evaluation in mice. Parasitol. Int. 2009;58:354–358. doi: 10.1016/j.parint.2009.07.006. [DOI] [PubMed] [Google Scholar]
- Ceballos L., Elissondo C., Sanchez Bruni S., Denegri G., Lanusse C., Alvarez L. Comparative performance of flubendazole and albendazole in cystic echinococcosis: ex vivo activity, plasma/cysts disposition and efficacy in infected mice. Antimicrob. Agents Chemother. 2011;55:5861–5867. doi: 10.1128/AAC.05105-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceballos L., Moreno L., Torrado J., Lanusse C., Alvarez L. Exploring flubendazole formulations for use in sheep. Pharmacokinetic evaluation of a cyclodextrin-based solution. BMC Vet. Res. 2012;8(1):71. doi: 10.1186/1746-6148-8-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceballos L., Mackenzie C., Geary T., Alvarez L., Lanusse C. Exploring the potential of flubendazole in filariasis control: evaluation of the systemic exposure for different pharmaceutical preparations. PLoS Negl. Trop. Dis. 2014 doi: 10.1371/journal.pntd.0002838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalgaard L. Comparison of minipig, dog, monkey and human drug metabolism and disposition. J. Pharmacol.Toxicol. Methods. 2015 doi: 10.1016/j.vascn.2014.12.005. [DOI] [PubMed] [Google Scholar]
- EMEA . Veterinary Medicines Evaluation Unit, Committee for Veterinary Medicinal Products; London, UK: 1997. The European Agency for the Evaluation of Medicinal Products. Flubendazol.http://www.ema.europa.eu/docs/en_GB/document_library/Maximum_Residue_Limits_-_Report/2009/11/WC500014288.pdf EMEA/MRL/267/97-FINAL. Summary Report 2. [Google Scholar]
- Evrard B., Chiap P., DeTullio F., Ghalmid F., Piela G., Van Heesa T., Crommen J., Losson B., Delattre L. Oral bioavailability in sheep of albendazole from a suspension on and from a solution containing hydroxypropyl-β-cyclodextrin. J. Control Release. 2002;85:45–50. doi: 10.1016/s0168-3659(02)00270-5. [DOI] [PubMed] [Google Scholar]
- Garcıa J., Bolas F., Torrado J. Bioavailability and efficacy characteristics of two different oral liquid formulations of albendazole. Int. J. Pharm. 2003;250:351–358. doi: 10.1016/s0378-5173(02)00559-8. [DOI] [PubMed] [Google Scholar]
- Geary T., Woo K., McCarthy J., Mackenzie C., Horton J., Prichard R., de Silva N., Olliaro P., Lazdins-Helds J., Engels D., Bundy D. Unresolved issues in anthelmintic pharmacology for helminthiases of humans. Int. J. Parasitol. 2010;40:1–13. doi: 10.1016/j.ijpara.2009.11.001. [DOI] [PubMed] [Google Scholar]
- Gibaldi M., Perrier D. second ed. Marcel Dekker; USA: 1982. Pharmacokinetics; pp. 45–109. Revised and Expanded. New York. [Google Scholar]
- Hennessy D., Al i D., Sillince J. The effect of a short-term reduction in feed on the pharmacokinetics and efficacy of albendazole in sheep. Aust. Vet. J. 1995;72:29–30. doi: 10.1111/j.1751-0813.1995.tb03472.x. [DOI] [PubMed] [Google Scholar]
- Lanusse C., Gascon L., Prichard R. Comparative plasma disposition kinetics of albendazole, fenbendazole, oxfendazole and their metabolites in adult sheep. J. Vet. Pharmacol. Ther. 1995;18:196–203. doi: 10.1111/j.1365-2885.1995.tb00578.x. [DOI] [PubMed] [Google Scholar]
- Lloberas M., Alvarez L., Entrocasso C., Virkel G., Lanusse C., Lifschitz A. Measurement of ivermectin concentrations in target worms and host gastrointestinal tissues: influence of the route of administration on the activity against resistant Haemonchus contortus in lambs. Exp. Parasitol. 2012 doi: 10.1016/j.exppara.2012.04.014. [DOI] [PubMed] [Google Scholar]
- Loftsson T., Duchene D. Historical Perspectives. Cyclodextrins and their pharmaceutical applications. Int. J. Pharm. 2007;329:1–11. doi: 10.1016/j.ijpharm.2006.10.044. [DOI] [PubMed] [Google Scholar]
- Mackenzie C., Geary T. Flubendazole: a candidate macrofilaricide for lymphatic filariasis and onchocerciasis field programs. Expert. Rev. Anti. Infect Ther. 2011;9:497–501. doi: 10.1586/eri.11.30. [DOI] [PubMed] [Google Scholar]
- Maté L., Virkel G., Lifschitz A., Ballent M., Lanusse C. Hepatic and extra-hepatic metabolic pathways involved in flubendazole biotransformation in sheep. Biochem. Pharmacol. 2008;76:773–783. doi: 10.1016/j.bcp.2008.07.002. [DOI] [PubMed] [Google Scholar]
- Moreno L., Alvarez L., Mottier L., Virkel G., Sanchez Bruni S., Lanusse C. Integrated pharmacological assessment of flubendazole potential for use in sheep: disposition kinetics, liver metabolism and parasite difussion ability. J. Vet. Pharmacol. Ther. 2004;27:299–308. doi: 10.1111/j.1365-2885.2004.00616.x. [DOI] [PubMed] [Google Scholar]
- Muller R.H., Schmidt S., Buttle I., Akkar A., Schmitt J., Bromer S. SolEmuls(R)-novel technology for the formulation of i.v. emulsions with poorly soluble drugs. Int. J. Pharm. 2004;269:293–302. doi: 10.1016/j.ijpharm.2003.09.019. [DOI] [PubMed] [Google Scholar]
- Rigter I., Schipper H., Koopmans R., van Kan H., Frijlink H., Kager P., Guchelaar H. Relative bioavailability of three newly developed albendazole formulations: a randomized crossover study with healthy volunteers. Antimicrob. Agents Chemother. 2004;48:1051–1054. doi: 10.1128/AAC.48.3.1051-1054.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suarez G., Alvarez L., Castells D., Correa O., Fagiolino P., Lanusse C. Comparative drug systemic exposure and clinical efficacy against resistant nematodes in lambs treated with different albendazole formulations. J. Vet. Pharmacol. Ther. 2011;34:557–564. doi: 10.1111/j.1365-2885.2011.01274.x. [DOI] [PubMed] [Google Scholar]
- Taylor M., Hoerauf A., Bockarie M. Lymphatic filariasis and onchocerciasis. Lancet. 2010;376:1175–1185. doi: 10.1016/S0140-6736(10)60586-7. [DOI] [PubMed] [Google Scholar]
- Toutain P., Bousquet-Mélou A. Bioavailability and its assessment. J. Vet. Pharmacol. Ther. 2004;27:455–466. doi: 10.1111/j.1365-2885.2004.00604.x. [DOI] [PubMed] [Google Scholar]
- Urbizu L., Confalonieri A., Sanchez Bruni S., Lanusse C., Alvarez L. Nematodicidal activity of flubendazole and its reduced metabolite on a murine model of Trichinella spiralis infection. Chemotherapy. 2012;58:295–298. doi: 10.1159/000342924. [DOI] [PubMed] [Google Scholar]
- WHO Programmes 2013. http://www.who.int/apoc/onchocerciasis/status/en/ Available at:
- Wu Z., Tucker I., Razzak M., Yang L., McSporran K., Medlicott N. Absorption and tissue tolerance of ricobendazole in the presence of hydroxypropyl-β-cyclodextrin following subcutaneous injection in sheep. Int. J. Pharm. 2010;397:96–102. doi: 10.1016/j.ijpharm.2010.07.002. [DOI] [PubMed] [Google Scholar]
- Zahner H., Schares G. Experimental chemotherapy of filariasis: comparative evaluation of the efficacy of filaricidal compounds in Mastomys coucha infected with Litomosoides carinii, Acanthocheilonema viteae, Brugia malayi and B. pahangi. Acta Trop. 1993;52:221–266. doi: 10.1016/0001-706x(93)90010-9. [DOI] [PubMed] [Google Scholar]