Abstract
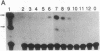
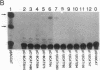
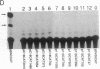
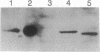
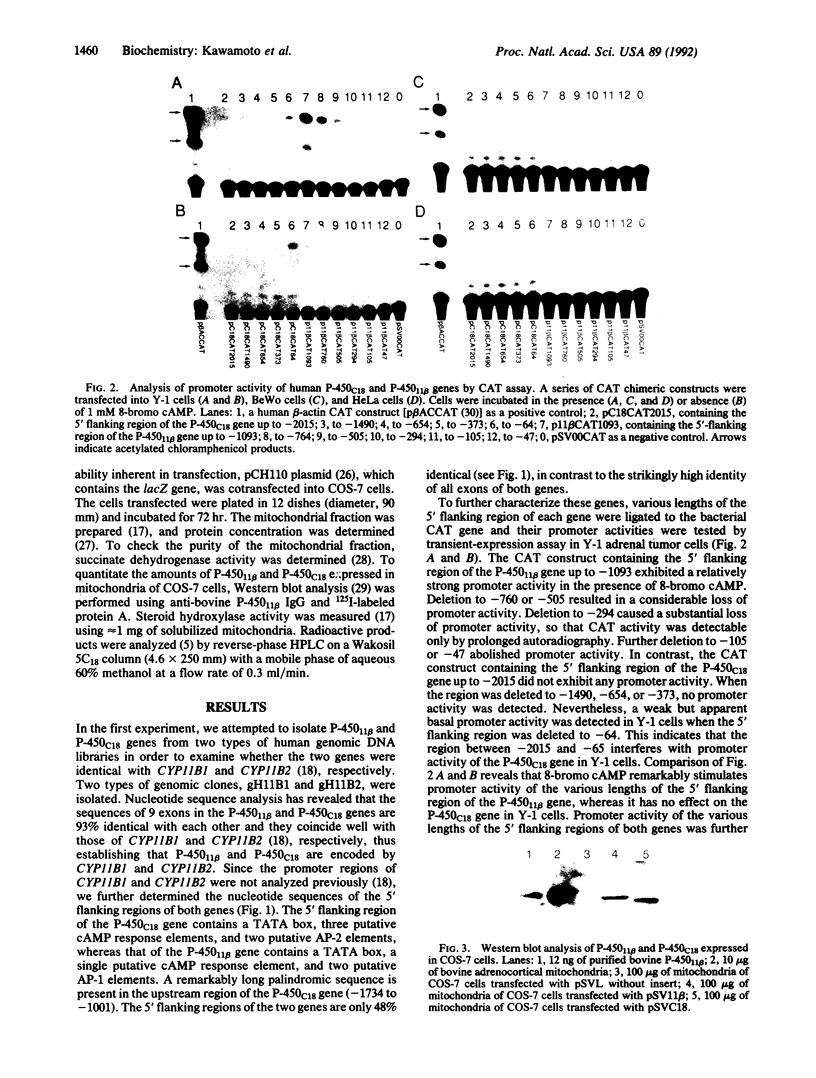
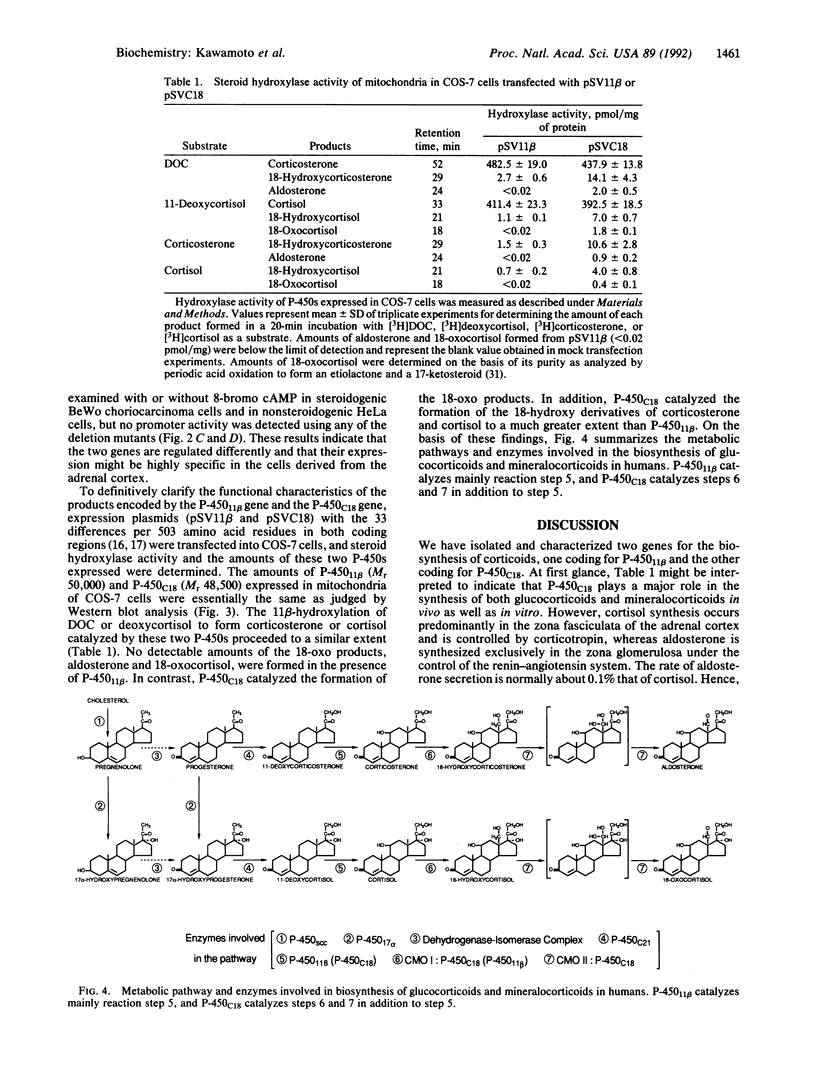
A gene encoding steroid 18-hydroxylase (P-450C18) was isolated from a human genomic DNA library. It was identified as CYP11B2, which was previously postulated to be a pseudogene or a less active gene closely related to CYP11B1, the gene encoding steroid 11 beta-hydroxylase (P-45011 beta) [Mornet, E., Dupont, J., Vitek, A. & White, P. C. (1989) J. Biol. Chem. 264, 20961-20967]. The nucleotide sequence of the promoter region of the P-450C18 gene is strikingly different from that of the P-45011 beta gene, although the sequences of their exons are 93% identical. The transient expression in Y-1 adrenal tumor cells of CAT constructs with a series of deletion mutants of promoter regions of both genes indicated that the two genes are regulated differently. P-450C18 as expressed in COS-7 cells exhibits steroid 18-hydroxylase activity to catalyze the synthesis of aldosterone and 18-oxocortisol and exhibits steroid 11 beta-hydroxylase activity as well. In contrast, P-45011 beta as expressed in the cultured cells exhibits steroid 11 beta-hydroxylase activity exclusively but fails to catalyze the synthesis of aldosterone and 18-oxocortisol. These results indicate that P-45011 beta and P-450C18 are products of two different genes and that the former participates in the synthesis of glucocorticoids whereas the latter participates in the synthesis of mineralocorticoids in humans.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Araki E., Shimada F., Shichiri M., Mori M., Ebina Y. pSV00CAT: low background CAT plasmid. Nucleic Acids Res. 1988 Feb 25;16(4):1627–1627. doi: 10.1093/nar/16.4.1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Chan C. K., Ulick S. Anomalous oxidative cleavage of the side chain of 18-oxocortisol and its tetrahydro metabolite. J Steroid Biochem. 1989 Oct;33(4A):605–611. doi: 10.1016/0022-4731(89)90048-4. [DOI] [PubMed] [Google Scholar]
- Curnow K. M., Tusie-Luna M. T., Pascoe L., Natarajan R., Gu J. L., Nadler J. L., White P. C. The product of the CYP11B2 gene is required for aldosterone biosynthesis in the human adrenal cortex. Mol Endocrinol. 1991 Oct;5(10):1513–1522. doi: 10.1210/mend-5-10-1513. [DOI] [PubMed] [Google Scholar]
- Hall C. V., Jacob P. E., Ringold G. M., Lee F. Expression and regulation of Escherichia coli lacZ gene fusions in mammalian cells. J Mol Appl Genet. 1983;2(1):101–109. [PubMed] [Google Scholar]
- Kawainoto T., Mitsuuchi Y., Ohnishi T., Ichikawa Y., Yokoyama Y., Sumimoto H., Toda K., Miyahara K., Kuribayashi I., Nakao K. Cloning and expression of a cDNA for human cytochrome P-450aldo as related to primary aldosteronism. Biochem Biophys Res Commun. 1990 Nov 30;173(1):309–316. doi: 10.1016/s0006-291x(05)81058-7. [DOI] [PubMed] [Google Scholar]
- Kawamoto T., Makino K., Orita S., Nakata A., Kakunaga T. DNA bending and binding factors of the human beta-actin promoter. Nucleic Acids Res. 1989 Jan 25;17(2):523–537. doi: 10.1093/nar/17.2.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamoto T., Mitsuuchi Y., Toda K., Miyahara K., Yokoyama Y., Nakao K., Hosoda K., Yamamoto Y., Imura H., Shizuta Y. Cloning of cDNA and genomic DNA for human cytochrome P-45011 beta. FEBS Lett. 1990 Sep 3;269(2):345–349. doi: 10.1016/0014-5793(90)81190-y. [DOI] [PubMed] [Google Scholar]
- Lauber M., Muller J. Purification and characterization of two distinct forms of rat adrenal cytochrome P450(11) beta: functional and structural aspects. Arch Biochem Biophys. 1989 Oct;274(1):109–119. doi: 10.1016/0003-9861(89)90421-9. [DOI] [PubMed] [Google Scholar]
- Lieberman S., Greenfield N. J., Wolfson A. A heuristic proposal for understanding steroidogenic processes. Endocr Rev. 1984 Winter;5(1):128–148. doi: 10.1210/edrv-5-1-128. [DOI] [PubMed] [Google Scholar]
- Marusic E. T., Mulrow P. J. Stimulation of aldosterone biosynthesis in adrenal mitochondria by sodium depletion. J Clin Invest. 1967 Dec;46(12):2101–2108. doi: 10.1172/JCI105697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meuli C., Müller J. A potassium-induced mitochondrial protein related to aldosterone biosynthesis. Am J Physiol. 1983 Nov;245(5 Pt 1):E449–E456. doi: 10.1152/ajpendo.1983.245.5.E449. [DOI] [PubMed] [Google Scholar]
- Mitani F., Shimizu T., Ueno R., Ishimura Y., Izumi S., Komatsu N., Watanabe K. Cytochrome P-45011 beta and P-450scc in adrenal cortex: zonal distribution and intramitochondrial localization by the horseradish peroxidase-labeled antibody method. J Histochem Cytochem. 1982 Oct;30(10):1066–1074. doi: 10.1177/30.10.6813370. [DOI] [PubMed] [Google Scholar]
- Mornet E., Dupont J., Vitek A., White P. C. Characterization of two genes encoding human steroid 11 beta-hydroxylase (P-450(11) beta). J Biol Chem. 1989 Dec 15;264(35):20961–20967. [PubMed] [Google Scholar]
- Nebert D. W., Gonzalez F. J. P450 genes: structure, evolution, and regulation. Annu Rev Biochem. 1987;56:945–993. doi: 10.1146/annurev.bi.56.070187.004501. [DOI] [PubMed] [Google Scholar]
- Nebert D. W., Nelson D. R., Adesnik M., Coon M. J., Estabrook R. W., Gonzalez F. J., Guengerich F. P., Gunsalus I. C., Johnson E. F., Kemper B. The P450 superfamily: updated listing of all genes and recommended nomenclature for the chromosomal loci. DNA. 1989 Jan-Feb;8(1):1–13. doi: 10.1089/dna.1.1989.8.1. [DOI] [PubMed] [Google Scholar]
- Neumann E., Schaefer-Ridder M., Wang Y., Hofschneider P. H. Gene transfer into mouse lyoma cells by electroporation in high electric fields. EMBO J. 1982;1(7):841–845. doi: 10.1002/j.1460-2075.1982.tb01257.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogishima T., Mitani F., Ishimura Y. Isolation of aldosterone synthase cytochrome P-450 from zona glomerulosa mitochondria of rat adrenal cortex. J Biol Chem. 1989 Jul 5;264(19):10935–10938. [PubMed] [Google Scholar]
- Ogishima T., Shibata H., Shimada H., Mitani F., Suzuki H., Saruta T., Ishimura Y. Aldosterone synthase cytochrome P-450 expressed in the adrenals of patients with primary aldosteronism. J Biol Chem. 1991 Jun 15;266(17):10731–10734. [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomatsu S., Kobayashi Y., Fukumaki Y., Yubisui T., Orii T., Sakaki Y. The organization and the complete nucleotide sequence of the human NADH-cytochrome b5 reductase gene. Gene. 1989 Aug 15;80(2):353–361. doi: 10.1016/0378-1119(89)90299-0. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ULICK S., GAUTIER E., VETTER K. K., MARKELLO J. R., YAFFE S., LOWE C. U. AN ALDOSTERONE BIOSYNTHETIC DEFECT IN A SALT-LOSING DISORDER. J Clin Endocrinol Metab. 1964 Jul;24:669–672. doi: 10.1210/jcem-24-7-669. [DOI] [PubMed] [Google Scholar]
- Ulick S., Chu M. D. Isolation and identification of an endogenous metabolite of 18-oxocortisol from human urine. J Steroid Biochem. 1987 Jul;28(1):89–94. doi: 10.1016/0022-4731(87)90129-4. [DOI] [PubMed] [Google Scholar]
- Ulick S. Diagnosis and nomenclature of the disorders of the terminal portion of the aldosterone biosynthetic pathway. J Clin Endocrinol Metab. 1976 Jul;43(1):92–96. doi: 10.1210/jcem-43-1-92. [DOI] [PubMed] [Google Scholar]
- Wada A., Okamoto M., Nonaka Y., Yamano T. Aldosterone biosynthesis by a reconstituted cytochrome P-45011 beta system. Biochem Biophys Res Commun. 1984 Feb 29;119(1):365–371. doi: 10.1016/0006-291x(84)91660-7. [DOI] [PubMed] [Google Scholar]
- Yanagibashi K., Haniu M., Shively J. E., Shen W. H., Hall P. The synthesis of aldosterone by the adrenal cortex. Two zones (fasciculata and glomerulosa) possess one enzyme for 11 beta-, 18-hydroxylation, and aldehyde synthesis. J Biol Chem. 1986 Mar 15;261(8):3556–3562. [PubMed] [Google Scholar]