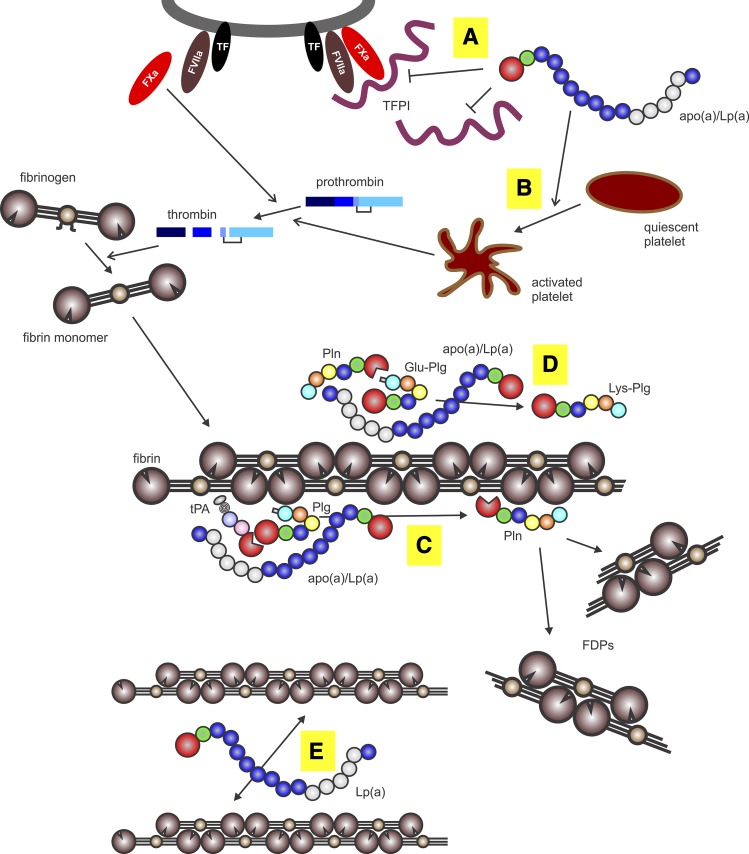
Fig. 3.
Procoagulant and antifibrinolytic mechanisms of apo(a). The apo(a) binds to either free or cell-bound TFPI (A), thereby promoting prothrombin activation by Factor Xa formed by the tissue factor (TF)-Factor VIIa complex. The apo(a) promotes platelet aggregation and release of granule contents through the thrombin receptor (B), promoting both platelet plug formation and enhanced prothrombin activation. Thrombin formed from prothrombin cleaves fibrinopeptides (A, B) from soluble fibrinogen to form fibrin monomers, which then polymerize to form insoluble fibrin. The apo(a) forms a ternary complex with fibrin, plasminogen (Plg), and tPA to prevent activation of plasminogen to form plasmin (Pln) (C). Plasmin proteolyzes fibrin to form soluble fibrin degradation products (FDPs). The apo(a) prevents plasmin-mediated conversion of Glu-plasminogen to Lys-plasminogen (D), the latter of which is a better substrate for tPA. Lp(a) alters fibrin structure to yield thinner and more dense fibers, potentially by binding to the αC domain of fibrin and, hence, preventing lateral association of fibrin fibrils (E).