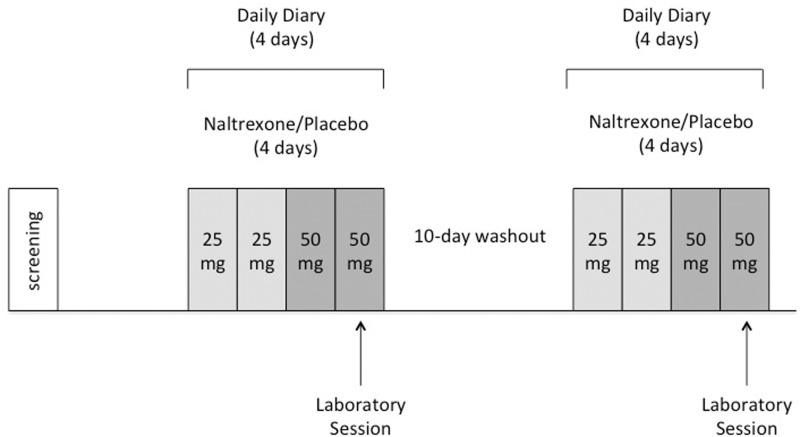
Figure 1.
Overview of procedures. After screening, participants completed a randomized, double-blind crossover study with the opioid-antagonist, naltrexone. Participants took four administrations of naltrexone, (25 mg on days 1 and 2 followed by 50 mg on days 3 and 4) as well as four administrations of placebo and completed two laboratory sessions (one on day 4 of naltrexone and one on day 4 of placebo), separated by a 10-day washout period. Daily diary reports were taken on all study drug days.