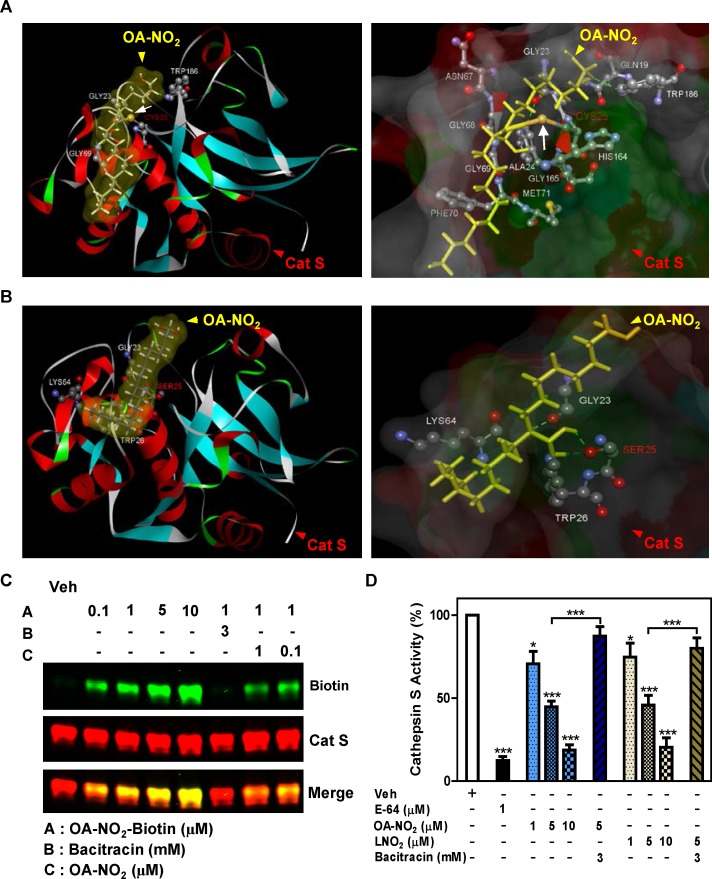
Fig 3. NFAs inhibit Cat S enzymatic activity by S-alkylation.
(A-C) Binding of OA-NO2 to Cat S was modeled in silico and evaluated in vitro. (A) Schematic representation showing covalent interaction of OA-NO2 with Cat S Cys25 residue. (B) Schematic representation showing absence of any covalent interaction of OA-NO2 with Cat S Cys25Ser mutation. (Left panels) Cat S is shown as a flat ribbon colored according to secondary structure; OA-NO2 (yellow) is shown as a stick model and interacting amino acids are shown in scaled ball-and-stick representation. (Right panels) Cat S is shown with interacting amino acid residues as a scaled ball-and-stick model with labels and OA-NO2 (yellow) as a stick model. In both figures the covalent bond is indicated by white arrow and hydrogen bonds are indicated by green dotted lines. (C) Human recombinant Cat S in active form (200 ng) was incubated for 30 min with the indicated compounds. Following incubation, S-alkylation of Cat S by biotin-labeled OA-NO2 in test samples was assessed by Western blotting under non-reducing conditions. (D) Human recombinant Cat S in active form (6 μU/μl) was incubated for 1 h with the indicated compounds and enzyme activity determined by cleavage of fluorescent substrate peptide. Data are representative of three independent experiments with n = 4/group. *P < 0.5, ***P < 0.001.