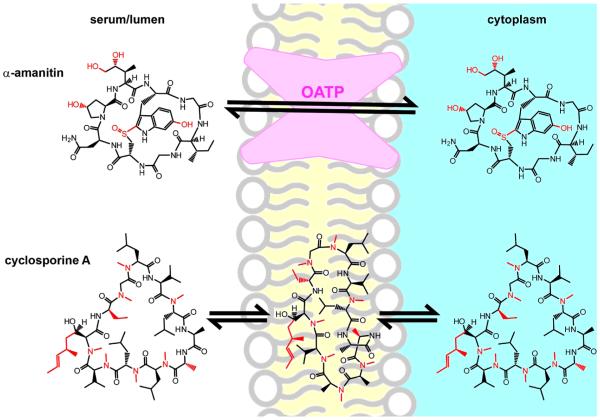
Figure 1.
“To be steady as a rock and always trembling.”144 α-Amanitin and cyclosporine A provide contrasting lessons from natural products. Both are head-to-tail cyclic peptides, and deviations from the 20 proteinogenic amino acids are shown in red. α-Amanitin is locked into a single conformation by virtue of a sulfone-indole intramolecular cross-link. This protects it from proteolytic degradation despite having a largely unmodified peptide backbone and appears to promote gut absorption and transport into liver cells by organic anion transport proteins (OATPs).12 Cyclosporine, by contrast, survives digestive proteases by virtue of its highly N-methylated backbone. It can change conformations in order to form intramolecular hydrogen bonds in nonpolar environments.23 This is hypothesized to promote passive diffusion through plasma membranes.