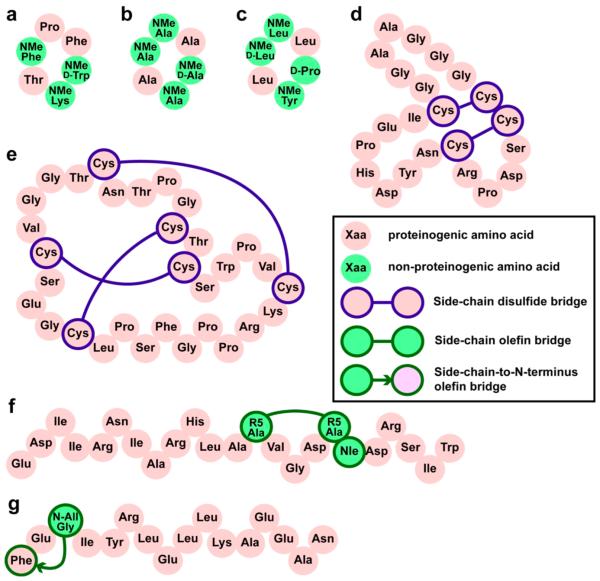
Figure 2.
Engineered peptides with high bioactivity and/or bioavailability. These constrained peptides vary greatly in size and hydrophobicity and employ different chemical cross-links, cyclizations, and folding topologies. (a) Somatostatin mimic with ~60 nM binding affinity to human somatostatin receptors sst2 and sst5. This compound permeates Caco-2 monolayers and has 7% oral bioavailability in rats.36 (b) Caco-2-penetrant cyclic peptide scaffold found by Kessler, Hoffman, and co-workers.135 (c) Cyclic peptide scaffold found by Lokey and co-workers to have 28% oral bioavailability in mice.24 (d) Cyclized α-conotoxin that targets GABAB receptors and acts as an analgesic. Head-to-tail cyclization resulted in oral bioavailablility as judged by effects on pain-related phenotypes in rats.56 (e) Grafted analogue of kalata B that acts as an orally bioavailable analgesic that targets bradykinin receptors.63 (f) Stapled helix of Bid that is able to slow proliferation of leukemia xenografts.95 (g) Hydrogen-bond-surrogate helix that can penetrate cells and inhibit Ras.109