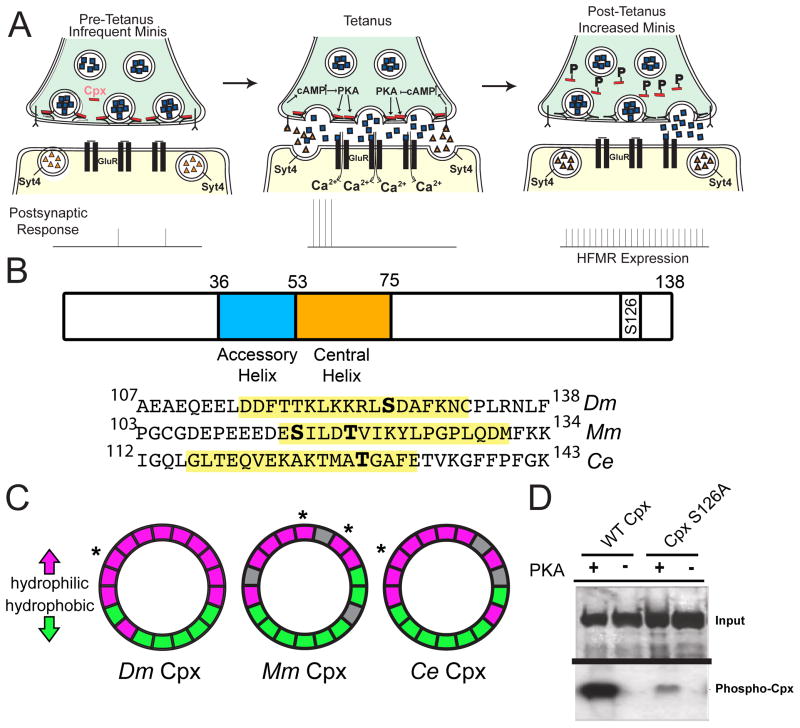
Figure 4.
Cpx is a substrate for PKA phosphorylation. (A) Proposed retrograde Syt 4-dependent signaling pathway that is activated by strong stimulation. Postsynaptic Ca++ elevation following stimulation drives release of a retrograde signal that activates the presynaptic PKA pathway. PKA is hypothesized to phosphorylate Cpx, altering its clamping properties. In addition, PKA may phosphorylate additional targets to facilitate structural growth. (B) Structure of Drosophila (Dm) Cpx and the location of the predicted PKA phosphorylation site (S126) in the C-terminus. The N-terminus and C-terminus flank the indicated accessory and central helix domains. The alignment of C-terminal regions of Dm Cpx, mouse (Mm) Cpx I, and C. elegans (Ce) Cpx is shown below. Predicted amphipathic helix domains are highlighted in yellow, and predicted phosphorylation sites indicated in bold. S115 of Mm Cpx I is phosphorylated by protein kinase CK2 (Shata et al., 2007). T119 of Mm Cpx I is a predicted substrate for PKC, and T129 of Ce Cpx is a predicted substrate for AKT (Wong et al., 2007). (C) Helical wheel diagrams of the amphipathic region for Dm Cpx, Mm Cpx I, and Ce Cpx. Hydrophilic resides are indicated in magenta, hydrophobic residues in green, and nonpolar and uncharged residues in grey. Predicted phosphorylation sites are indicated by *. (D) CpxS126A exhibited greatly decreased [32P] incorporation compared to WT Cpx (CpxS126A 32P incorporation was reduced to 35.2 ± 10.6 % relative band intensity of WT Cpx 32P incorporation (n=4)). Recombinant control Cpx (WT) and phosphoincompetent CpxS126A were incubated with [32P]ATP and recombinant active PKA. Phosphorylation was visualized by autoradiography after proteins were separated by SDS-PAGE (bottom panel). Gels were also stained with Coomassie blue to assay protein loading (top panel).