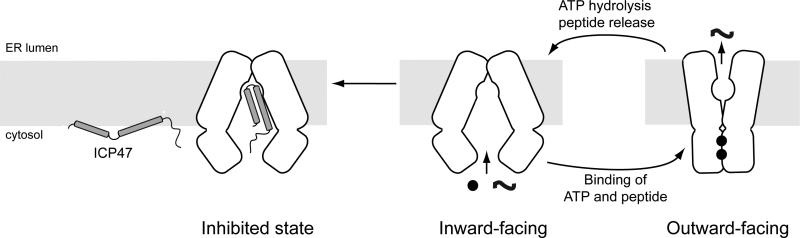
Figure 4. ICP47 precludes peptide binding and traps TAP in an inward-facing conformation.
TAP functions via alternating access, cycling between two major conformations. In the absence of substrates, the transporter rests in an inward-facing state in which the two NBDs are separated and the translocation pathway is exposed to the cytosol. Upon association of substrates and ATP, the transporter undergoes a conformational change that reorients the TMDs and positions ATP at a closed NBD dimer interface for hydrolysis. ATP hydrolysis releases the substrate and resets the transporter to the resting state. ICP47 binds TAP stabilizes the inward-facing conformation.