Figure 5.
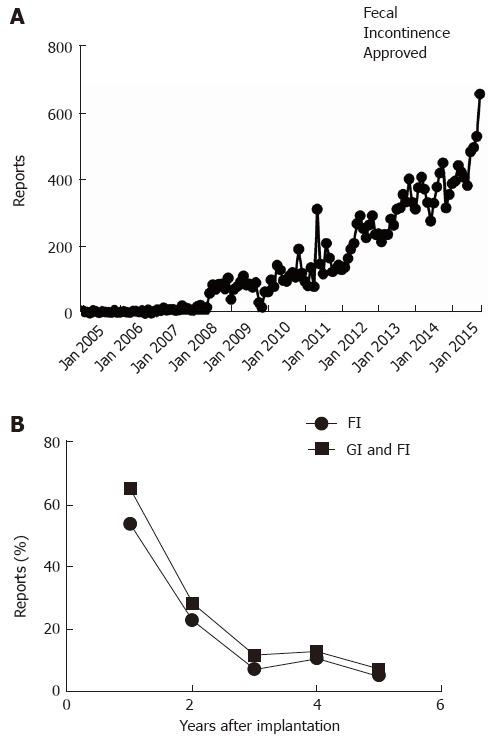
Manufacturer and user device experience databank. A: Reports about adverse events related to therapy with Interstim are plotted as a function of month of posting by the Federal Drug Administration; B: The lower panel depicts the percentage of reports mentioning gastrointestinal problems (squares) or specifically fecal incontinence (circles) as indication for sacral neurostimulation.
