Figure 6.
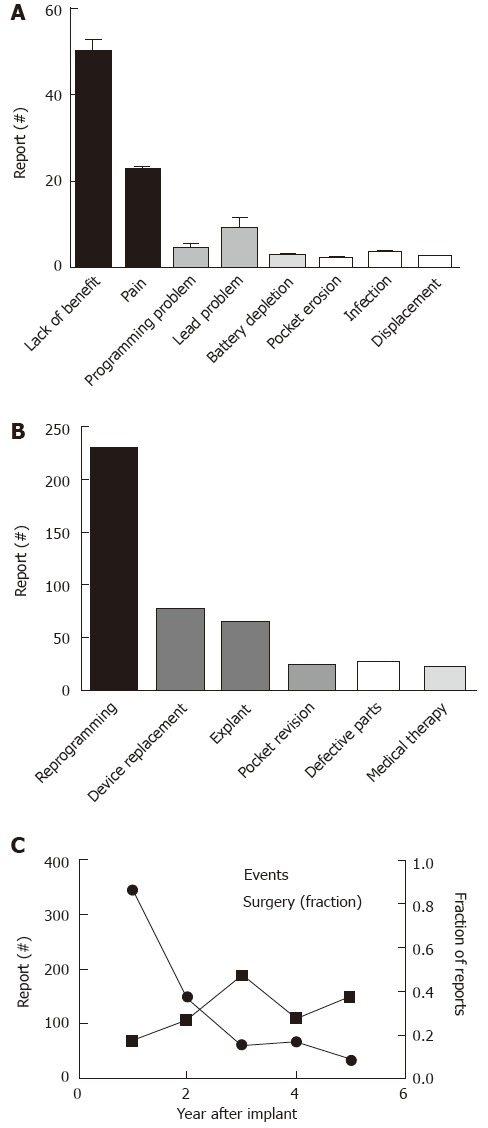
Concerns and interventions related to Interstim treatment and described in manufacturer and user device experience. A: Depicts the number of reported events sorted based on the primary concerns; B: Shows the main corrective steps taken (“Defective Parts” refers to intra-operative problems with device components requiring exchange); C: The proportions of operatively treated interventions is plotted in relation to the overall number of reported adverse events.
