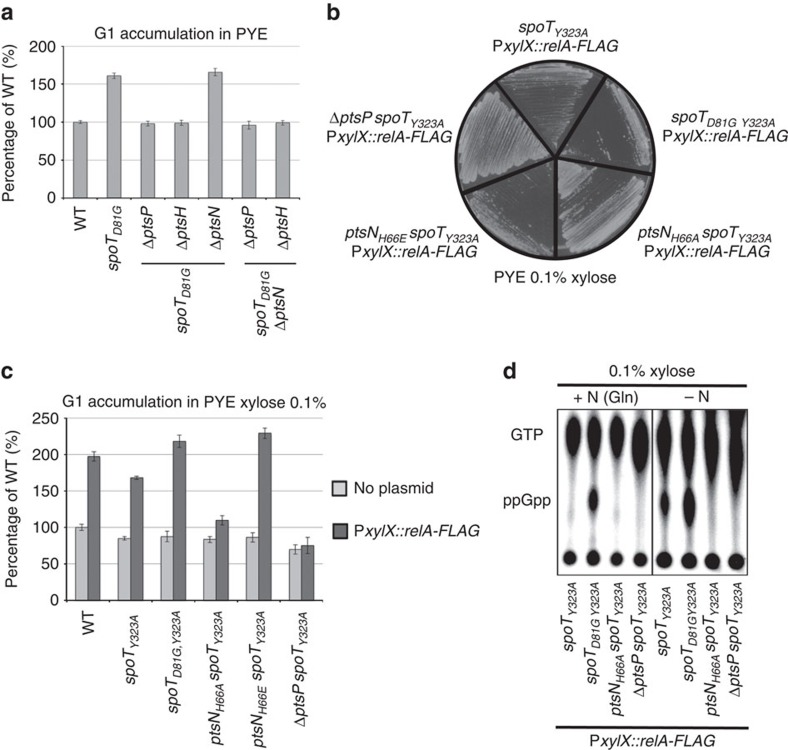
Figure 7. EIIANtr∼P inhibits the hydrolase activity of SpoT.
(a) spoTD81G is insensitive to the absence of EIIANtr. G1 proportion was measured in wild-type (WT; RH50), spoTD81G (RH1752), spoTD81G ΔptsP (RH1727), spoTD81G ΔptsH (RH2013), spoTD81G ΔptsN (RH1999), spoTD81G ΔptsN ΔptsP (RH2014) and spoTD81G ΔptsN ΔptsH (RH2015) grown in complex media (PYE) and normalized to the WT (100%). Error bars=s.d.; n=3. (b) The hydrolase activity of SpoT is required for growth on an artificial exogenous production of (p)ppGpp. Growth of spoTY323A, spoTD81G Y323A, ptsNH66AspoTY323A, ptsNH66EspoTY323A and ΔptsP spoTY323A expressing a truncated version of E. coli relA from the inducible xylX promoter (PxylX::relA-FLAG) on PYE medium supplemented with 0.1% of xylose. (c) Reduction of SpoT hydrolase activity led to a G1 extension on an artificial exogenous production of (p)ppGpp. Flow cytometry analysis to determine DNA content in asynchronous population of WT, spoTY323A, spoTD81G Y323A, ptsNH66AspoTY323A, ptsNH66EspoTY323A and ΔptsP spoTY323A with (dark grey bars) or without (light grey bars) PxylX::relA-FLAG in PYE medium supplemented with 0.1% of xylose. The data were normalized to the WT without PxylX::relA-FLAG (100%). Error bars=s.d.; n=3. (d) The hydrolase activity of SpoT is required to degrade (p)ppGpp in +N condition. Intracellular levels of (p)ppGpp detected by TLC after nucleotides extraction of spoTY323A, spoTD81G Y323A, ptsNH66AspoTY323A and ΔptsP spoTY323A containing PxylX::relA-FLAG in +N or −N medium supplemented with 0.1% xylose.