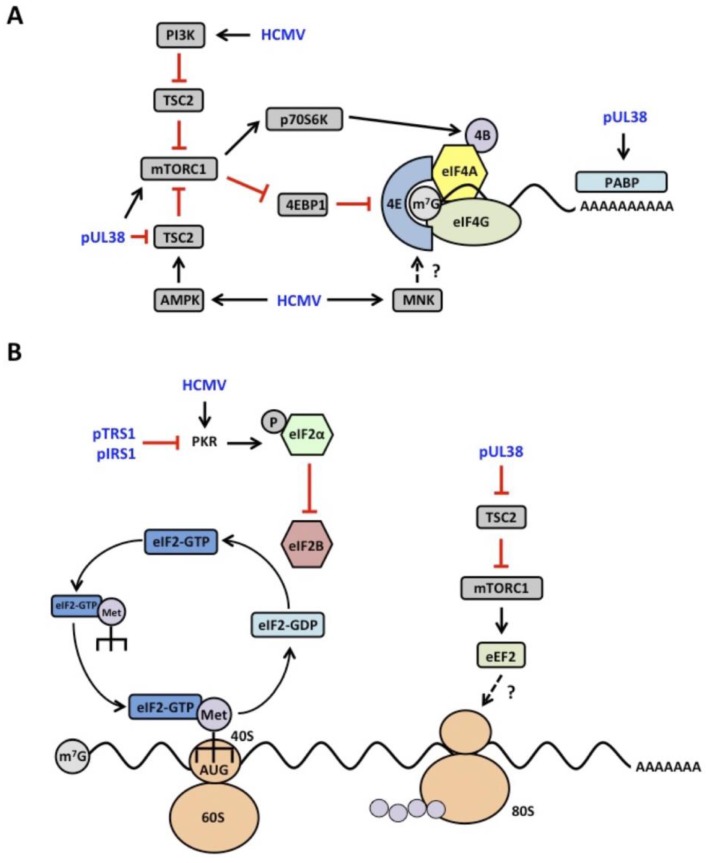
Figure 1.
Schematic of human cytomegalovirus (HCMV) manipulation of translation initiation and elongation. (A) HCMV stimulates eIF4F formation and activity through multiple mechanisms. Infection increases levels of eIF4F components (eIF4E, eIF4G, eIF4A) and poly(A) binding protein (PABP). HCMV also promotes eIF4F assembly by activating mammalian target of rapamycin complex 1 (mTORC1), which phosphorylates and inhibits the eIF4F antagonist 4EBP1. The HCMV UL38 protein prevents inactivation of mTORC1 by inhibiting the tuberous sclerosis complex (TSC). pUL38 also stimulates mTORC1 activity through a TSC2-independent mechanism. mTORC1 activates p70S6K, which phosphorylates eIF4B to increase eIF4A helicase activity. HCMV infection activates the PI3K pathway to promote mTORC1 activation, and may also regulate translation through activation of MNK kinases (B) Translation initiation and elongation are maintained during HCMV infection. Inhibition of eIF2α phosphorylation ensures the regeneration of ternary complexes and continued rounds of translation initiation. Levels of eEF2 increase during infection through a UL38-dependent mechanism and may promote translation during HCMV infection.