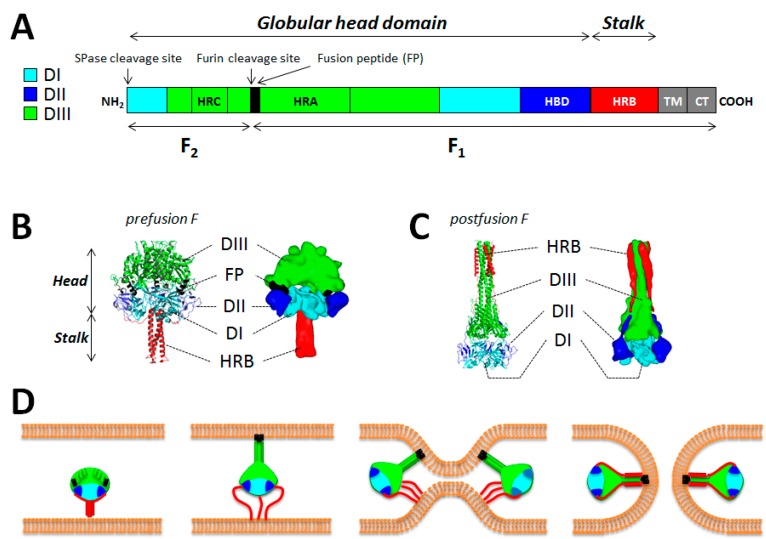
Figure 1.
Structure/function of the measles virus (MeV) fusion protein. (A) The important functional domains of F are color-coded. The F1 and F2 subunits are shown. While the DI (light blue), DII (dark blue) and DIII (green) domains define the globular head domain, the HRB region defines the stalk. TM: transmembrane domain; CT: cytoplasmic tail; HRA-C: heptad repeat region A–C; FP: fusion peptide; HBD: H-binding domain; SPase: signal peptidase. (B and C) Structural differences between the pre and postfusion states of F. Images were obtained from homology models performed with the canine distemper virus (CDV) F sequence (strain A75/17) and based on the atomic coordinates of the human parainfluenza virus type 5 (hPIV5) F prefusion state (PDB 2B9B) and hPIV3 F postfusion state (PDB 1ZTM). DI, DII and DIII are color-coded as indicated in (A). Images were generated using either the PyMol (high resolution) or Sculptor (low resolution) software packages (D) Model of membrane fusion induced by measles virus F. Once activated by H, prefusion F may disengage from H interaction. Then the HRB region of the stalk may melt, which consequently may enable the 11 segments positioned in the globular head to rearrange into a long three-helix bundle (3HB) coiled-coil. This would in turn propel the fusion peptide at the top of the 3HB and allow its insertion into the target plasma membrane. This prehairpin intermediate then collapses, which brings both membranes into close proximity. Finally, F may reach the highly stable postfusion state, which carries the six-helix bundle (6HB) formed by HRA and HRB segments. This stage is thought to correlate with membrane merging and ensuing fusion pore opening. This model likely requires the concerted action of more than one F molecules. This model has been described first by Yin and colleagues [79,86].