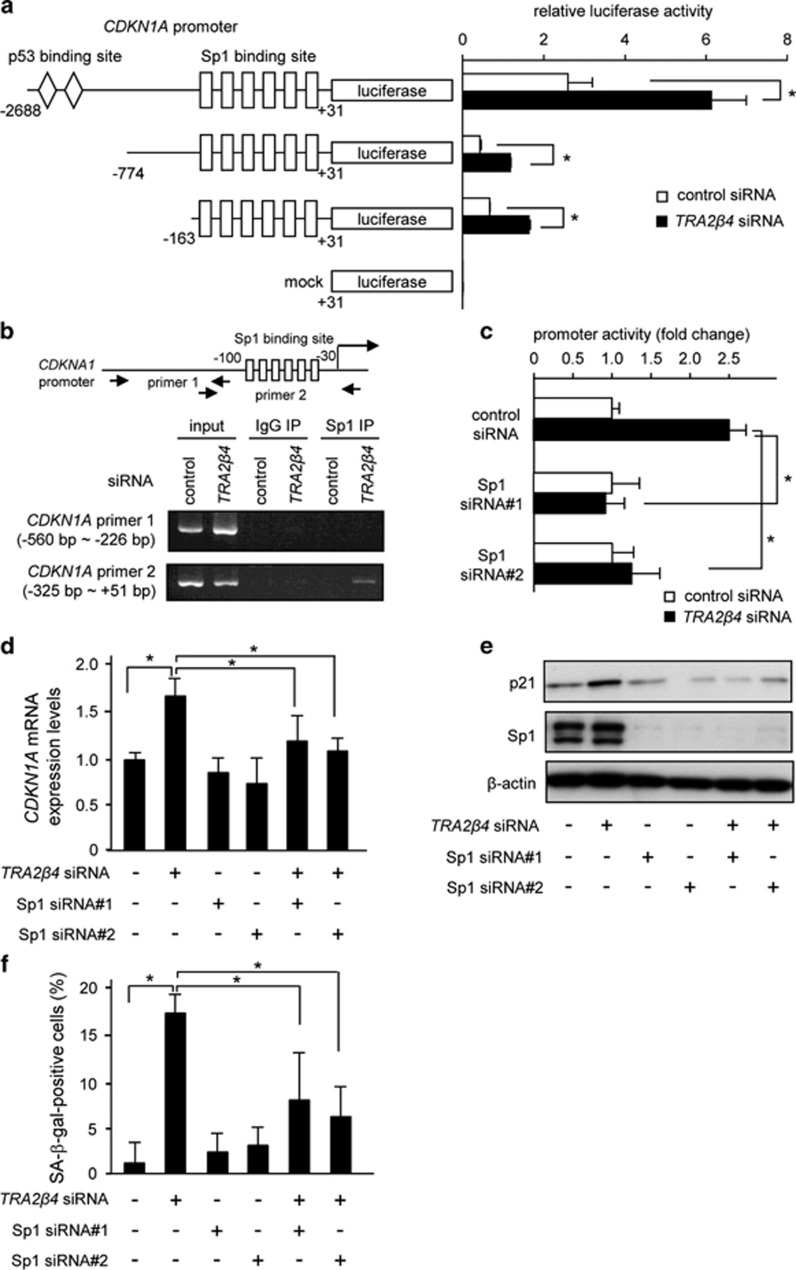
Figure 5.
TRA2β4 modifies promoter activity of the CDKN1A gene. (a) Twenty-four hours after transfection with 10 nm TRA2β4 or control siRNA, HCT116 cells were transiently transfected with luciferase reporter plasmids driven by −2688/+31, −774/+31 or −163/+31 bp promoter fragments of CDKN1A for 24 h. Luciferase activities in these cells were measured using the Dual-Luciferase Reporter Assay System. *Significantly decreased compared with control siRNA-treated cells (P<0.05 by analysis of variance (ANOVA) and Bonferroni test). (b) After treatment with TRA2β4 or control siRNA for 48 h, HCT116 cells were subjected to chromatin immunoprecipitation (ChIP) assays. Formaldehyde-crosslinked nuclear extracts were immunoprecipitated with an anti-Sp1 antibody or normal rabbit IgG (IgG). PCR was performed using an input nuclear chromatin fraction as a template (input). Specific PCR products corresponding to the region of the CDKN1A promoter containing the Sp1-binding sites were amplified and separated by agarose gel electrophoresis followed by ethidium bromide staining. (c) After treatment with 10 nm Sp1, TRA2β4 or control siRNA for 24 h, HCT116 cells were transiently transfected with the luciferase plasmid (pGL3-CDKN1A −163/+31) for 24 h. Luciferase activities in these cells were measured using the Dual-Luciferase Reporter Assay System. Values are means±s.d. (n=4). *Significantly different (P<0.05 by ANOVA and Bonferroni test). (d and e) After HCT116 cells were treated with TRA2β4 and/or Sp1 siRNA nos 1/2 as indicated for 24 h, expression levels of CDKN1A mRNA and p21 were analyzed by qPCR and western blotting. (f) After silencing of TRA2β4 and/or Sp1 nos 1/2, the cells were stained with SA-β-gal and then one hundred cells per individual sample in three independent fields were measured. *Significantly different (P<0.05 by ANOVA and Bonferroni test).