Abstract
Objective:
Many controversies exist regarding the extent of resection for insular gliomas and the timing of resection. Several techniques and adjuncts are used to maximize safety during resection of these tumors. We describe the use of indocyanine green (ICG) to identify the branches of the middle cerebral artery and discuss its utility to increase safety for resection for insular gliomas.
Materials and Methods:
Five patients with insular gliomas were surgically treated by the authors from June 2013 to June 2014. The patients presented with complaints of either a headache or recurring episodes of convulsions. All the patients were operated with the aid of neuronavigation and tractography. The long perforating branches of the middle cerebral artery course through the insula and pass onward to supply the corona radiata. It is essential to preserve these vessels to prevent postoperative neurological deficits. ICG (Aurogreen) was used to identify and preserve the long perforating arteries of the middle cerebral artery.
Results:
ICG dye correctly identified the long perforating branches of the middle cerebral artery and easily distinguished these vessels from the short perforating branches. All the branches of the middle cerebral artery that coursed through the tumor and had an onward course were preserved in all the patients. Only one patient developed a transient right sided hemiparesis that had improved at follow-up.
Conclusions:
Surgery for insular gliomas is challenging due to its location adjacent to eloquent areas, important white fiber tracts and the course of the middle cerebral artery within it. ICG is useful to identify and preserve the long perforating branches of the middle cerebral artery that course through the tumor and traverse onward to supply the corona radiata.
Keywords: Indocyanine green, insular glioma, surgery
Introduction
The insula also known as the “Island of Reil” lies hidden beneath the frontal, parietal and temporal opercula [Figure 1]. It has been a subject of great interest and has been said to have roles in motor, sensory, visceral, auditory, and speech functions. It is a site of various pathological processes such as gliomas, metastases, and vascular malformations. Approach to the insular region is riddled with difficulties due to its eloquent location and its close relation with the branches of the middle cerebral artery. Indocyanine green (ICG) has paved its way into vascular neurosurgery and is used routinely for aneurysms, arteriovenous malformations, and arterio-venous fistulae. However, its use in tumor surgery is limited. There are a few recent reports where ICG has been used to identify venous sinuses in meningioma surgery [Video].[1] We present five cases where ICG was used to identify branches of the middle cerebral artery during insular glioma surgery thereby allowing a safe surgical resection.
Figure 1.
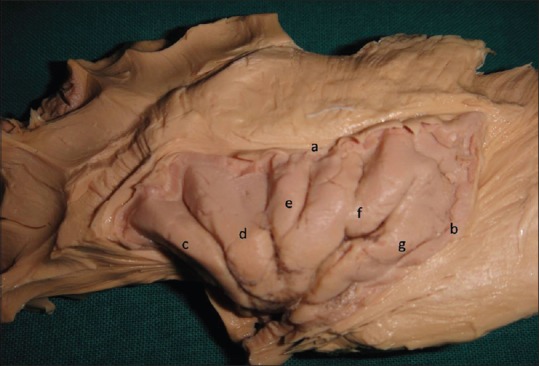
Cadaveric specimen showing the insular cortex. The frontal, temporal and parietal opercula have been removed and the white fiber tracts surrounding the insula dissected to display the hidden island. (a) Superior limiting periinsular sulcus, (b) posterior limiting periinsular sulcus, (c) anterior short insular gyrus, (d) middle short insular gyrus, (e) posterior short insular gyrus, (f) anterior long insular gyrus and (g) posterior long insular gyrus
Materials and Methods
From June 2013 to June 2014, five patients of insular gliomas who were treated surgically with the aid of ICG dye are presented. The clinical history, preoperative neurological status and postoperative neurological status were recorded. All patients underwent preoperative neuronavigation protocol magnetic resonance imaging (MRI) and diffusion tensor imaging. The images of the neuronavigation MRI and diffusion tensor imaging were fused and used for intraoperative planning and surgery. After the initial tumor dissection, intraoperatively, ICG dye was used to identify the branches of the middle cerebral artery and safeguard them.
Clinical features
All the patients presented with a history of headache and seizures. The duration of symptoms varied from 1 month to 1 year (average 3 months). The indications of surgery were headache not responding to conservative management or seizures in spite of anticonvulsant therapy. None of the patients had any neurological deficit.
Radiological features
In one patient, the tumor was on the dominant side and in four patients the tumor was located in the nondominant hemisphere. On MRI the tumors involved varying degrees of the insula and perisylvian areas [Figures 2 and 3]. The tumor was localized only to the insula in one patient, involved the temporal opercula mainly in one patient and both the operculae in three patients. There was uncal herniation and mass effect in four patients. The tumors were hypointense on T1-weighted imaging and hyperintense mainly on T2-weighted imaging. In two patients, mild inhomogeneous enhancement was seen and in three patients no enhancement was visualized. On diffusion tensor imaging, the fibers of the superior longitudinal fasciculus, uncinate fasciculus, and occipitofrontal fasciculus were displaced by the tumor. The descending motor pathways were pushed medially by the tumor. In none of the patients were any of the white fiber tracts infiltrated or destroyed. A diagnosis of low to intermediate grade glioma was made preoperatively in all the patients. The patients underwent MRI with BrainLab neuronavigation protocol and diffusion tensor imaging. The images of MRI and diffusion tensor imaging were fused on BrainLab workstation, and the surgical approach was planned.
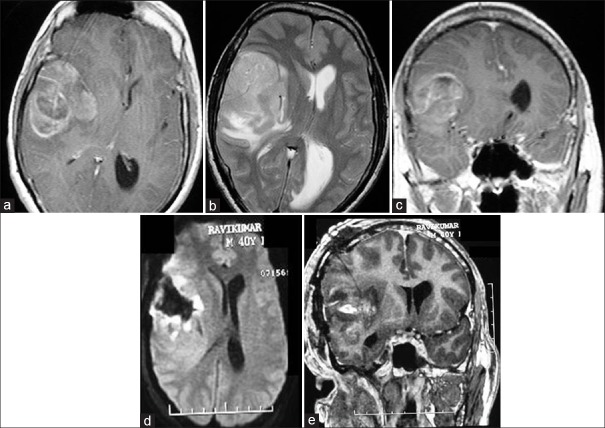
Figure 2.
Images of a 40-year-old male patient with headache and seizures. (a) T1-weighted axial contrast image showing the right insular glioma predominantly involving the frontal opercula. (b) T2-weighted axial image showing the tumor with mass effect. (c) Coronal contrast image showing the heterogeneously enhancing insular tumor. (d) Postoperative axial flair image showing resection of the tumor. (e) Postoperative coronal contrast image showing the resection
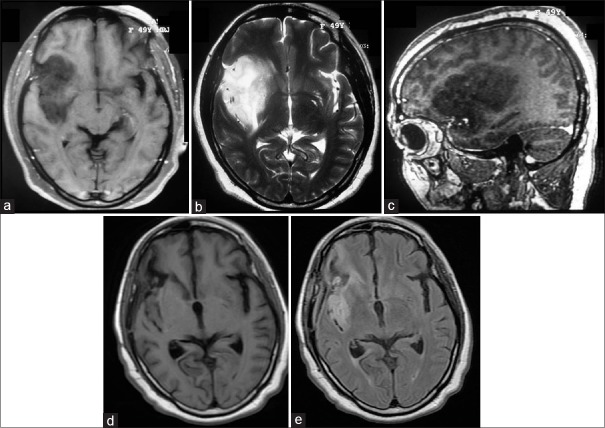
Figure 3.
Images of a 50-year-old female with headache. (a) T1-weighted axial image showing the hypointense lesion in the insular region. (b) T2-weighted image showing the insular glioma. (c) T1-weighted sagittal contrast image showing the nonenhancing lesion involving zones I and II primarily. (d) Delayed postoperative T1-weighted image showing resection of the tumor. (e) Delayed postoperative flair image showing excision of the tumor with radiation changes
Figure 4.
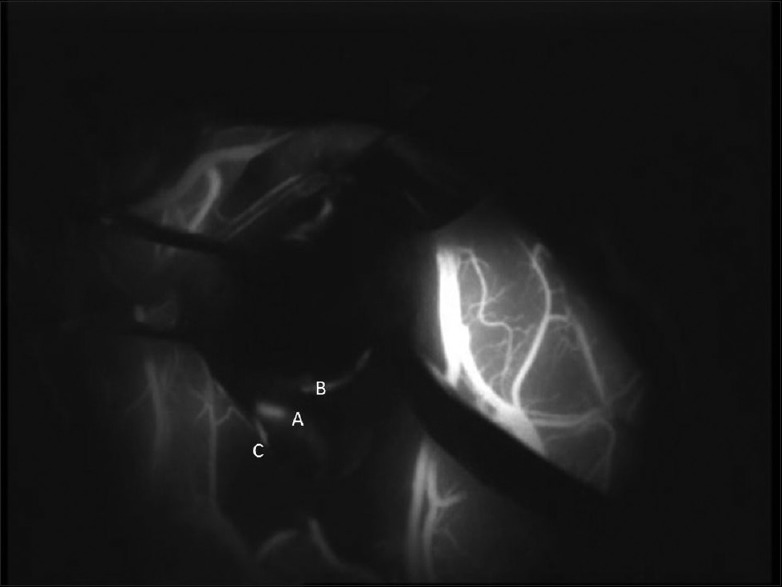
Intra-operative image after indocyanine green administration. The long perforating vessel which does not enter the tumor and courses onward can be clearly visualized. (a) M2 segment of the middle cerebral artery, (b) long perforating branch of the M2 segment, (c) M3 segment of the middle cerebral artery
Surgery
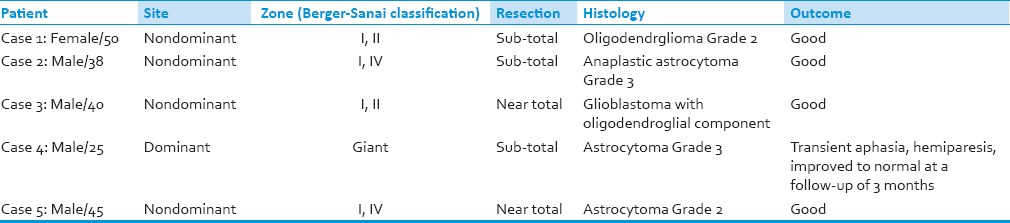
All patients were operated in the supine position with the head turned to the contralateral side. Under neuronavigation guidance, a question mark shaped incision was marked, and a fronto-temporal craniotomy was performed. The frontal and temporal opercula and the sylvian fissure were visualized. Either the transcortical or the trans-sylvian approaches were used for resection of the tumors. In patients with tumors localized only to the insula, a trans-sylvian approach was used and the sylvian fissure was exposed from distal to proximal. In patients with either a predominant frontal or temporal extension, the initial corticectomy was made in the noneloquent frontal or temporal brain. On the dominant side, the corticectomies were placed anteriorly to safeguard the Broca's and Wernicke's area. The corticectomy was also positioned such that the tracts visualized on the integrated diffusion tensor imaging were avoided. The tumor in the frontal and temporal region away from the insula was initially excised. Then the swollen insula was visualized. In patients where there was a purely insular tumor, after the opening of the sylvian fissure, the expanded insula was seen. In both the situations, the branches of the middle cerebral artery were identified. Tumor removal was then performed between the M2 branches of the middle cerebral artery safeguarding them. At this stage, it sometimes became difficult to identify the branches that ended in the insula and the tumor and those that coursed the insula making their way to the corona radiata. At this juncture, ICG (15–25 mg) was injected intravenously, and the insular region was visualized with the Flow 800 software (Carl Zeiss Meditec, Oberkochen, Germany) under the infrared camera of the Pentero microscope. The middle cerebral artery and its M2 branches were clearly visualized. The onward coursing branches were identified and preserved, and only those branches that were seen ending in the region of the tumor were coagulated. The tumor dissection was then continued medially up to the region of the extreme capsule and claustrum. Thus, a safe resection of the tumor was performed. In patient with a significant temporal extension a temporal lobectomy was additionally performed. In three patients, a subtotal resection was performed and in two patients a near total resection was performed [Table 1].
Table 1.
The demographic and tumor characteristics
Outcome/Results
We could preserve the branches of the middle cerebral artery in all our patients. Only one patient developed a postoperative neurological deficit. In this patient immediately postoperatively there was a right sided hemiparesis (Grade 2/5) with aphasia. Even in this patient, all the branches of the middle cerebral artery had been preserved. The patient improved within the next 5 days and at the time of discharge was verbalizing and had hemiparesis of Grade 3 in the upper limb and Grade 4 in the lower limb. At follow-up of 3 months, his hemiparesis and speech deficit had almost normalized. The histology was astrocytoma Grade 2 in one patient, oligodendroglioma Grade 2 in one patient, Grade 3 astrocytoma in two patients and glioblastoma with an oligodendroglial component in one patient. All patients were treated with adjuvant radiotherapy. There were no complications related to the use of ICG dye. At follow-up ranging from 9 to 21 months all the five patients were doing well.
Discussion
The insula is a mesocortical structure along with the temporal pole, caudal orbitofrontal cortex, cingulate and parahippocampal gyri. It connects the limbic region to the neocortex.[2,3,4,5] The insula or the fifth lobe of the brain lies hidden beneath the frontal, temporal, and parietal opercula. Three-dimensionally the insular shape represents an inverted pyramid with the apex lying at the anterior sylvian point beneath the pars triangularis of the inferior frontal gyrus. The insula is bounded by the anterior, superior and inferior periinsular sulci. The insular stem lies in the antero – basal portion of the insula near the insular apex. The limen insula lies within the insular stem. This is the landmark where the M1 segment of the middle cerebral artery becomes M2 by bifurcating into the superior and inferior trunks. It also represents the medial limit of resection of insular gliomas and has a close relation with the lateral lenticulostriate arteries. The insular cortex is made up of the short and long insular gyri. The anterior, middle, and posterior short insular gyri are present anteriorly and are separated from the anterior and posterior long insular gyri by the central insular sulcus. The extreme capsule, claustrum, external capsule, and the basal ganglia are located medially and deep to the insular cortex. The uncinate fasciculus runs deep to the anterior portion of the superior periinsular sulcus, and the posterior limb of the internal capsule runs deep to the posterior segment of the superior periinsular sulcus.[3] About 25% of low-grade gliomas and 10% of high-grade gliomas occur in the region of the insula.[6] In the initial phases of growth, these tumors limit themselves to the mesocortical and allocortical areas, respecting the neocortical areas, central nuclei, and ventricles.[2] Resection of insular gliomas is fraught with risks of postoperative deficits due to proximity to the extreme capsule, claustrum, external capsule, striatum, the internal capsule, and the uncinate fasciculus. The surgery is also technically challenging due to the branches of the middle cerebral artery running through the insula.
Insular gliomas were generally considered inoperable, until Yasargil et al., proposed a safe trans-sylvian approach to resect these tumors in 1992.[4] He classified insular and parainsular gliomas into four types. Insular gliomas have been recently classified based on the Berger-Sanai insular glioma classification system.[7] According to this system, the insula is divided into four zones. The insula is bisected into a horizontal plane by a line passing through the sylvian fissure. A perpendicular plane is then intersected with this at the level of the foramen of Monro. The resultant quadrants (anterior-superior, posterior-superior, posterior-inferior, and anterior-inferior) are designated zones I, II, III, and IV, respectively. The number of zones that they lie in designates the tumors. If the tumor occupies all four zones these insular gliomas are termed as giant.
In recent years, due to a better understanding of the anatomy and functions of the limbic system, paralimbic areas, and the insula, neurosurgeons have begun to venture into the insula for resection of pathological lesions involving the region.[2,5] Maximal safe tumor resection is believed by many to be the aim of surgery, but the role of aggressive surgery remains controversial and no class 1 evidence exists. A recent study suggests that the predilection of an insular glioma for malignant transformation may be reduced with aggressive cytoreduction.[8] Several technical adjuncts are available to help make the resection easier and safer. The use of cortical and subcortical motor mapping techniques with awake craniotomy, functional MRI, diffusion tensor imaging and intra-operative MRI have helped in improving the safety of resection of these tumors. In spite of all these available adjuncts aggressive resection of insular gliomas can still be riddled with complications, especially in the hands of a neurosurgeon unfamiliar with the anatomy of the region.
The M1 segment of the middle cerebral artery courses within the sylvian vallecula and the lateral lenticulostriate arteries arise from this segment. The M1 segment turns 90° at the limen insula and forms the genu where the artery bifurcates. The insula receives blood supply from about 75 to 104 perforating arteries that predominantly arise from the M2 segment of the middle cerebral artery.[9] The short perforating arteries supply the insular cortex, the medium perforating arteries also supply the claustrum and external capsule and the long perforating branches extend as far as the corona radiata and supply it. The short perforating arteries usually run within the tumor and can be coagulated safely. Deficits due to vascular injury can occur due to damage to the lateral lenticulostriate arteries and the long perforating branches of the M2 segment of the middle cerebral artery. The lateral lenticulostriate arteries supply the basal ganglia and the internal capsule. They usually arise as branches of the inferomedial portion of the M1 segment near the limen insula. Usually, these arteries do not supply the insula and can be identified early in the surgery. These arteries should be the medial limit of resection of the tumor. The long perforating arteries are branches of the M2 segment and run through the insula to go on to supply the corona radiata. They are located primarily in the posterior region of the insula most commonly on the posterior half of the central insular sulcus and on the long insular gyri. This region corresponds to zone II of the Berger-Sanai classification system.[10] It is absolutely critical to prevent injury to these vessels. It is difficult to identify these vessels intraoperatively sometimes, especially for a novice. They can be confused with the short perforating arteries that supply the insula and the tumor. ICG was used to identify the course of these long perforating vessels. A dose of 15–25 mg was used intravenously. The ICG was used in our cases after the initial resection of the tumor after the branches of the middle cerebral artery were visualized. It was mainly used to distinguish between vessels ending in the tumor from those which had an onward course further on to supply the fibers of the corona radiata.
ICG (C43H47N2NaO6S2) is a near-infrared fluorescent tricarbocyanine dye that is being applied in a wide range of neurosurgical cerebrovascular procedures. The technique of near infrared angiography allows real-time visualization of the blood flow through the blood vessels at the time of surgery with the help of the operating microscope. ICG is ideal due to its short half-life and acceptable safety profile. After injection, the dye binds to plasma proteins within 1 to 2 s and remains intravascular in conditions of normal vascular permeability. The dye is not metabolized by the body and is excreted by the liver with a plasma half-life of 150 s.[1]
Fluorescence emitting substances have been used to detect tumor margins during surgery and thus increase the extent of resection. ICG is regularly used to visualize vessels and their patency during aneurysm and arteriovenous malformation surgery. It has also been used to detect venous anatomy during parasagittal meningioma surgery, to detect blood flow in tumor vasculature and to differentiate pituitary tumors from the normal gland.[1,11] We describe the use of ICG to detect normal vasculature within insular tumors thus preserving the vessels and preventing postoperative deficits. Iwasaki et al. used ICG to confirm the patency of these long insular arteries after temporary occlusion for motor evoked potential monitoring during resection of insular gliomas.[10]
Yasargil et al., used routine angiography to study the anatomy of the vessels before operating on tumors in the insular region.[4] Duffau described the use of computed tomography (CT) angiography to study the branches of the middle cerebral artery before surgery.[2] They even suggested integration of the CT angiography with image guidance software to study the vasculature. ICG provides a real-time visualization of the vessels amidst the tumor and also traces the course of the vessel outside the insula. Thus, the long perforating arteries that supply the corona radiata can be identified and preserved. The technique is easy, quick and safe to perform.
Conclusions
ICG can be a useful adjunct during surgery for insular gliomas. It helps in identifying and preserving the long perforating branches of the middle cerebral artery and the lateral lenticulostriate arteries, thus preventing morbidity.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Video Avaiable: www.asianjns.org
References
- 1.Della Puppa A, Rustemi O, Gioffrè G, Rolma G, Grandis M, Munari M, et al. Application of indocyanine green video angiography in parasagittal meningioma surgery. Neurosurg Focus. 2014;36:E13. doi: 10.3171/2013.12.FOCUS13385. [DOI] [PubMed] [Google Scholar]
- 2.Duffau H. A personal consecutive series of surgically treated 51 cases of insular WHO grade II glioma: Advances and limitations. J Neurosurg. 2009;110:696–708. doi: 10.3171/2008.8.JNS08741. [DOI] [PubMed] [Google Scholar]
- 3.Rey-Dios R, Cohen-Gadol AA. Technical nuances for surgery of insular gliomas: Lessons learned. Neurosurg Focus. 2013;34:E6. doi: 10.3171/2012.12.FOCUS12342. [DOI] [PubMed] [Google Scholar]
- 4.Yasargil MG, von Ammon K, Cavazos E, Doczi T, Reeves JD, Roth P. Tumours of the limbic and paralimbic systems. Acta Neurochir (Wien) 1992;118:40–52. doi: 10.1007/BF01400725. [DOI] [PubMed] [Google Scholar]
- 5.Shah A, Jhawar SS, Goel A. Analysis of the anatomy of the Papez circuit and adjoining limbic system by fiber dissection techniques. J Clin Neurosci. 2012;19:289–98. doi: 10.1016/j.jocn.2011.04.039. [DOI] [PubMed] [Google Scholar]
- 6.Duffau H, Capelle L. Preferential brain locations of low-grade gliomas. Cancer. 2004;100:2622–6. doi: 10.1002/cncr.20297. [DOI] [PubMed] [Google Scholar]
- 7.Sanai N, Polley MY, Berger MS. Insular glioma resection: Assessment of patient morbidity, survival, and tumor progression. J Neurosurg. 2010;112:1–9. doi: 10.3171/2009.6.JNS0952. [DOI] [PubMed] [Google Scholar]
- 8.Duffau H. A new philosophy in surgery for diffuse low-grade glioma (DLGG): Oncological and functional outcomes. Neurochirurgie. 2013;59:2–8. doi: 10.1016/j.neuchi.2012.11.001. [DOI] [PubMed] [Google Scholar]
- 9.Türe U, Yasargil MG, Al-Mefty O, Yasargil DC. Arteries of the insula. J Neurosurg. 2000;92:676–87. doi: 10.3171/jns.2000.92.4.0676. [DOI] [PubMed] [Google Scholar]
- 10.Iwasaki M, Kumabe T, Saito R, Kanamori M, Yamashita Y, Sonoda Y, et al. Preservation of the long insular artery to prevent postoperative motor deficits after resection of insulo-opercular glioma: Technical case reports. Neurol Med Chir (Tokyo) 2014;54:321–6. doi: 10.2176/nmc.cr2012-0361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim EH, Cho JM, Chang JH, Kim SH, Lee KS. Application of intraoperative indocyanine green videoangiography to brain tumor surgery. Acta Neurochir (Wien) 2011;153:1487–95. doi: 10.1007/s00701-011-1046-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.