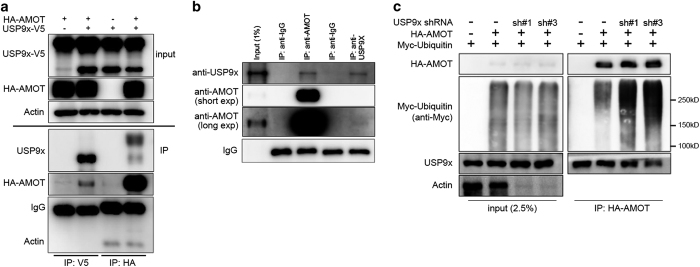
Figure 3.
USP9x regulates AMOT ubiquitylation. (a) Immunoblots of HEK293T cells transfected to express HA-AMOT and V5-tagged USP9x. Cell lysates were immunoprecipitated using anti-HA to pull down HA-p130-AMOT or with anti-V5 to pull down V5 AMOT. Blots were probed with anti-V5 to detect USP9x-V5 (input) and with anti USP9x (IP). Note that the V5 antibody pulls down predominantly the shorter form of USP9x. Both long and short forms of USP9x were recovered by IP with HA-AMOT. (b) Immunoblots of HEK293T cell lysates immunoprecipitated using anti-AMOT or anti-USP9x or with non-immune IgG as a control. Blots were probed with anti-AMOT and with anti-USP9x. The recovery of endogenous AMOT by IP was very efficient. However, recovery of UPS9x by IP was very weak, which limits the sensitivity of detection of AMOT by USP9x IP. (c) Immunoblots of HEK293T cells transfected to express HA-AMOT with Myc-tagged Ubiquitin, and shRNA vectors to deplete USP9x or control shRNAs. Cells were treated with MG132 to block proteasome activity. Lysates were immunoprecipitated with anti-HA to recover AMOT and blots were probed to detect Ubiquitin (anti-Myc), AMOT (anti-HA) or with anti-Actin to control for loading. Anti-USP9x was used to monitor the efficiency of shRNA-mediated depletion in the total cell lysate. Depletion of USP9x was efficient. Note that the total level of ubiquitylated protein in the cell was unchanged in the total cell lysates after USP9x depletion. IP, immunoprecipitation.