Abstract
Most morbidity associated with malignancy in long-term renal transplant recipients is due to cutaneous squamous cell carcinoma (SCC). Previously identified measures to stratify SCC risk have limited use, however. We hypothesized that an increased proportion of senescent, terminally differentiated CD8+ T cells would identify renal transplant recipients at elevated SCC risk. Peripheral blood lymphocytes were isolated from 117 stable transplant recipients at high risk of SCC and analyzed phenotypically by flow cytometry. Participants were followed up prospectively for SCC development. The predictive value of variables was assessed using Cox regression. Age at transplant and enrollment, dialysis duration, and previous disease were predictive of SCC development during follow-up. Previously published clinical phenotype-based risk scores lost predictive value with the removal of age as a covariate. The percentage of CD57-expressing CD8+ T cells was the strongest immunologic predictor of future SCC and correlated with increasing CD8+ T cell differentiation. We dichotomized participants into those with a majority (CD57hi) and a minority (CD57lo) of CD8+ T cells expressing CD57; CD57hi participants were more likely to develop SCC during follow-up (hazard ratio, 2.9; 95% confidence interval, 1.0 to 8.0), independent of potential confounders, and tended to develop earlier recurrence. The CD57hi phenotype was stable with time and associated with increasing age and cytomegalovirus seropositivity. Our results show that the CD57hi phenotype is a strong predictor of SCC development and recurrence in this cohort of long-term, high-risk renal transplant recipients. This information may allow identification of recipients who may benefit from intensive dermatologic screening and immunosuppression reduction.
Keywords: cancer, risk factors, renal transplantation, immunology, cytomegalovirus
Non melanoma skin cancer (NMSC), 80% due to squamous cell carcinoma (SCC), represents 90% of malignancy seen in long-term renal transplant recipients (RTRs), with an incidence up to 200 times that of the general population.1 NMSC incidence rises with cumulative immunosuppression and ultraviolet (UV) exposure: cumulative incidence is up to 50% in the UK and 90% in Australia at 20 years post-transplant.2,3 Post-transplant SCC occurs at a younger age and is more aggressive than in nontransplant cohorts, with 30% of SCC recurring within 1 year and up to 8% of disease associated with metastasis.2,4–8 The median survival from diagnosis of metastasis is 3 years.7,9
Attempts have been made to identify RTRs at increased SCC risk.10 Three risk scores, developed in different cohorts, utilize clinical phenotype, including skin type, age and sun exposure.2,3,11 Immunologic factors such as increased number of CD4+CD25hiCD127loFoxP3+ regulatory T cells (Treg) may have predictive value for SCC development; however, the predictive value is relatively short term and predicts only high-risk SCC recurrence.12,13 The performance of clinical and immunologic predictive markers has not been directly compared.
Following antigenic stimulation, most naive T cells undergo clonal expansion, acquiring effector function, with subsequent apoptosis. A minority differentiate into long-lived memory T cells.14 Effector memory (TEm) T cells circulate through peripheral lymphoid tissue, and display rapid cytolytic function ex vivo, while central memory (TCm) cells circulate through secondary lymphoid tissue and lack immediate cytotoxic capability.15 TEm cells acquire maximal cytolytic ability and re-acquire CD45RA expression upon terminal differentiation (termed TEMRA), marked by gain of CD57 and loss of CD27 and CD28 expression.16,17 CD57 may be used to identify T cells that have undergone repeated rounds of antigenic stimulation, possessing the shortest telomeres among T cell populations, with impaired proliferation and cytokine production upon polyclonal in vitro stimulation, and are considered senescent.17,18 An increased proportion of terminally differentiated CD8+ cells and an inverted CD4/CD8 ratio is associated with impaired protective immunity to viral pathogens and vaccination and increased mortality in the elderly.19–21
As part of a prospective, longitudinal study assessing the immune phenotype of long-term RTRs, we hypothesized that the accumulation of terminally differentiated/senescent CD8+ T cells may impair antiviral and antitumor responses and enable the identification of RTRs at increased risk of SCC. We compared the performance of this marker with previously identified clinical and immunologic predictors of future malignancy.
Results
Participant Recruitment and Baseline Phenotype
Sixty-five eligible RTRs with a history of post-transplant SCC (referred to as RTRSCC) were identified, of which 63 were approached and 59 participated. Seventy-two eligible RTRs without a previous history of SCC (RTRNo) were approached and 58 were recruited. RTRSCC were significantly older and more likely to report a history of any malignancy in a parent or sibling (Table 1). Fifteen percent of participants received induction therapy at time of transplant, and four-fifths had received a period of dialysis prior to transplantation. Of the three clinical phenotype scores examined, only the Urwin score was significantly increased in RTRSCC.
Table 1.
Clinical phenotype of study participants at enrolment
| Variable | RTRSCC (n=59) | RTRNo (n=58) | P Value |
|---|---|---|---|
| Caucasiana | 58 (98) | 55 (95) | 0.36 |
| Male | 41 (71) | 39 (67) | 0.21 |
| Age (years) | 66 (58–74) | 61 (55–67) | 0.03 |
| Body mass index (kg/m2) | 25.2 (21.7–28.2) | 26.2 (23.2–29.7) | 0.09 |
| Age at first transplant (years) | 43 (31–52) | 40 (32–49) | 0.30 |
| Dialysis before transplant | 44/56 (79) | 44/56 (79) | 1.00 |
| Duration of dialysis (months) | 14 (7–36) | 13 (7–25) | 0.53 |
| Total duration of immunosuppression (months)b | 283 (208–353) | 249 (203–312) | 0.17 |
| Received more than one transplant | 13 (22) | 10 (17) | 0.56 |
| Induction therapy | 0.53 | ||
| None | 51 (86) | 48 (83) | |
| Anti-CD25 | 5 (9) | 6 (10) | |
| Thymoglobulin | 3 (5) | 4 (7) | |
| Previously treated for rejection | 32/54 (59) | 22/54 (41) | 0.05 |
| Number of times treated | 2 (1–3) | 2 (1–3) | 0.42 |
| Number of HLA-ABDR mismatches | 2 (2–4) | 2 (1–4) | 0.71 |
| Serum creatinine (mmol/l) | 117 (90–168) | 126 (106–161) | 0.27 |
| eGFR (ml/min per 1.73 m2) | 50.9 (35.5–64.0) | 44.2 (34.0–57.5) | 0.19 |
| Cytomegalovirus IgG seropositive | 40 (68) | 36 (62) | 0.52 |
| Immunosuppression: | |||
| Calcineurin inhibitor | 49 (83) | 47 (81) | 0.78 |
| Azathioprine | 46 (78) | 38 (66) | 0.14 |
| Mycophenolate | 4 (7) | 9 (16) | 0.15 |
| Prednisolone | 24 (41) | 22 (38) | 0.76 |
| Sirolimus | 4 (7) | 2 (3) | 0.35 |
| Daily dosage (mg/kg)c | |||
| Ciclosporin | 1.90 (1.60–2.90) | 2.05 (1.58–2.55) | 0.81 |
| Tacrolimus | 0.06 (0.06–0.10) | 0.06 (0.04–0.08) | 0.25 |
| Azathioprine | 0.85 (0.55–1.13) | 0.80 (0.58–1.20) | 0.85 |
| Mycophenolate | 11.9 (7.9–15.1) | 14.6 (13.0–19.0) | 0.28 |
| Prednisolone | 0.07 (0.06–0.08) | 0.06 (0.05–0.07) | 0.12 |
| Trough level (ng/ml)d | |||
| Ciclosporin | 76 (54–104) | 83 (59–100) | 0.80 |
| Tacrolimus | 7.4 (6.3–9.4) | 6.5 (5.7–7.8) | 0.32 |
| Smoker (>1 pack-year history) | 20 (34) | 23 (40) | 0.52 |
| Previous non-NMSC malignancy | 5 (9) | 11 (19) | 0.10 |
| History of malignancy in parents/siblings | 31 (53) | 20 (35)& | 0.04 |
| Chronic UV exposure [5] | 38 (64) | 32 (55) | 0.31 |
| Fitzpatrick skin type | 0.07 | ||
| Type I–II | 27 (47) | 17 (29) | |
| Type III–VI | 29 (51) | 41 (71) | |
| Type V–VI | 1 (2) | 0 | |
| Nonbrown eye color | 44 (75) | 37 (65) | 0.26 |
| Harden risk score [12] | 5.26 (4.2–6.16) | 4.96 (4.35–5.82) | 0.43 |
| Urwin risk score [6] | 2 (1–3) | 1.5 (0–2) | 0.01 |
| Harwood risk score [5] | 4 (2–4) | 3 (2–4) | 0.10 |
Continuous variables are reported as median (IQR) while categorical variables are reported as number (percentage of column). Where there are missing data, a denominator of number of known values in that column is given.
Two participants were adopted and thus were unable to provide a family history.
Including pretransplant immunosuppression where appropriate.
Daily dosage is reported as a median (IQR) across RTRs receiving that medication.
If below limit of detection, was given value 1 unit below this (i.e. if <30 ng/ml, given value 29 ng/ml).
There were no significant differences in immune phenotype between groups, performed at enrolment (Table 2). Notably, there was no increase in the number or percentage of γδ T cells or Treg in RTRSCC. The distribution of CD57 expression on CD8+ T cells was nonparametric and bimodally distributed, so RTRs were dichotomized on the basis of a majority (i.e. >50%, referred to as CD57hi) or minority (CD57lo) of CD57+ cells within the CD8+ population, as the cut-off level of 50% approximated to the population mean and nadir between peaks (data not shown).
Table 2.
Immune phenotype of study participants at enrolment
| Variable | RTRSCC | RTRNo | P Value |
|---|---|---|---|
| Lymphocyte count | 1135 (863–1658) | 1360 (1040–1728) | 0.15 |
| Monocyte count | 500 (410–635) | 475 (413–600) | 0.58 |
| Total (CD3+) T cell count | 945 (649–1339) | 1157 (785–1467) | 0.25 |
| Gamma-delta T cell count | 33 (14–79) | 38 (13–97) | 0.77 |
| Gamma-delta T cell percentage | 2.6 (1.5–5.5) | 3.0 (1.2–6.7) | 0.83 |
| CD4+ T cell count | 567 (431–700) | 666 (474–836) | 0.13 |
| CD4+ T cell percentage | 48 (39–61) | 49 (40–60) | 0.79 |
| Treg (CD4+CD69-CD25hiCD127loFoxP3+) cell count | 16 (9–22) | 13 (9–19) | 0.51 |
| Treg percentage (of CD4+ population) | 2.5 (1.8–3.6) | 2.3 (1.7–3.0) | 0.19 |
| CD57+ percentage (of CD4+ population) | 11 (7–28) | 11 (6–18) | 0.35 |
| CD8+ T cell count | 275 (132–410) | 284 (179–510) | 0.20 |
| CD8+ T cell percentage | 22 (15–31) | 24 (16–32) | 0.61 |
| CD8+CD57+ T cell count | 162 (48–282) | 146 (77–310) | 0.65 |
| Naïve CD8+ T cell (percentage of CD8+) | 11 (4–33) | 15 (5–34) | 0.96 |
| CD8+ TEm (percentage of CD8+) | 24 (14–39) | 22 (12–43) | 0.90 |
| CD8+ TEMRA (percentage of CD8+) | 54 (29–73) | 42 (20–74) | 0.40 |
| CD8+ TCm (percentage of CD8+) | 1 (0.4–4) | 1 (0.3–3) | 0.50 |
| CD57+ percentage (of CD8+ population) | 67 (39–76) | 53 (36–70) | 0.18 |
| >50% of CD8+ T cells CD57+ (‘CD57hi’ phenotype) | 36 (61) | 29 (50) | 0.21 |
| NK cell (CD3-CD56+) count | 30 (16–109) | 43 (13–121) | 0.97 |
| NK cell percentage | 3.5 (1.4–9.7) | 3.1 (1.0–9.1) | 0.70 |
| CD19+ B cell count | 19 (8–44) | 19 (12–40) | 0.45 |
| CD19+ B cell percentage | 1.7 (0.9–3.7) | 1.8 (0.9–2.9) | 0.38 |
Continuous variables are reported as median (IQR) while categorical variables are reported as number (percentage of column). Counts are reported as cells per microliter of blood, while percentages are reported as a fraction of the lymphocyte population, unless specified otherwise. Groups were compared by Mann–Whitney test.
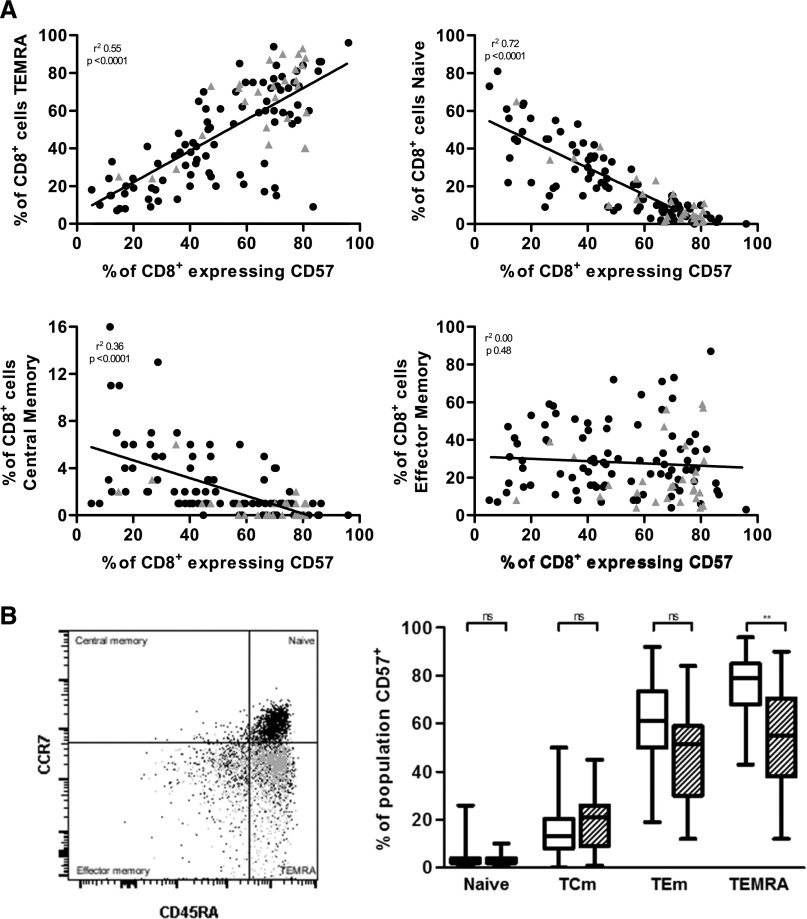
We next looked at CD57 as an immunosenescence marker. The percentage of CD57-expressing CD8+ T cells demonstrated positive correlation with the proportion of TEMRA CD8+ cells in peripheral blood, and negative correlation with the percentage of TCm and naive CD8+ T cells (Figure 1A) and CD4/CD8 ratio (r2 0.21, P<0.001). CD57-expressing cells were predominantly located in the CCR7– effector compartment, in particular the TEMRA subset (Figure 1B). The proportion of CD57-expressing CD8+ T cells showed significant correlation with CD4+ T cell expression of CD57 (r2 0.65, P<0.001). As with the CD8+ compartment, CD57 expression within the CD4+ compartment inversely correlated with the proportion of naive and TCm CD4+ (data not shown). Thus increasing expression of CD57 correlates with other previously demonstrated markers of increasing immunosenescence and T cell differentiation.
Figure 1.
Increasing CD57 expression correlates with increasing differentiation of the CD8+ T cell compartment. (A) Correlation of CD57 expression on CD8+ T cells with CD8 populations. Grey triangles indicate those RTRs who developed SCC during follow-up. (B) Representative gating indicating the location of all CD8+ T cells (black) and CD8+CD57+ T cells (grey) within CD8 subsets, and frequency of CD57-expressing cells among CD8 populations (stratified by cytomegalovirus seropositivity: non-shaded bars indicate seropositive RTRs). ns, not significant. **P<0.01 using the Kruskal–Wallis with post hoc Dunn’s tests.
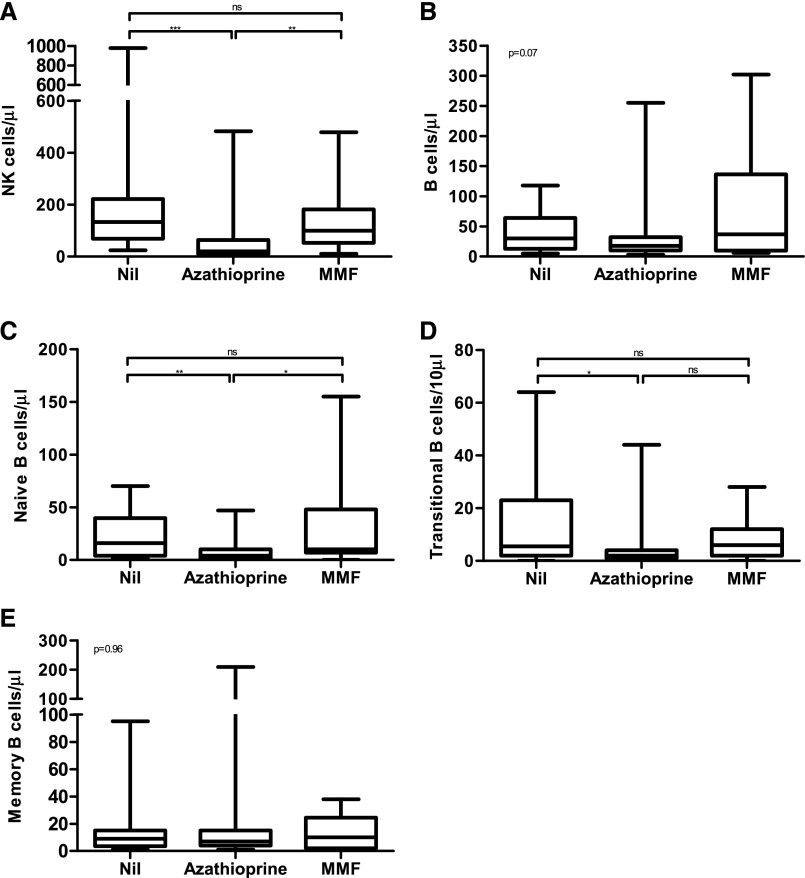
Previous studies have suggested that natural killer (NK) and B cell phenotype can predict SCC development.12,13 However, the interpretation of NK and B cell data was hampered by the effect of azathioprine upon both populations (Figure 2). Azathioprine may impact bone marrow B cell output, as both transitional and naive B cell numbers were significantly reduced in RTRs receiving azathioprine, with sparing of the memory compartment. Mycophenolate mofetil did not have this effect. We did not further analyze these populations due to this. Monocyte and T cell numbers and the proportion of CD57-expressing CD8+ T cells were unaltered when stratified by antimetabolite or calcineurin inhibitor use, although we saw a reduction in those taking steroids (Supplemental Figure 2). The proportion taking steroids and the mean dose did not differ between those in the CD57hi and CD57lo arms.
Figure 2.
Use of azathioprine, but not MMF, is associated with a reduction in transitional and naive B cells and NK cells. NK and B cell population numbers in peripheral blood stratified by antimetabolite use. (A) Total NK (CD3+CD56-) cells per µl of blood. (B) Total B cells (CD3-CD19+) per µl of blood; (C) Naive B cells (CD19+CD27-IgD+) per µl of blood. (D) Transitional B cells (CD19+CD24hiCD38hi) per 10 µl of blood. (E) Memory B cells (CD19+CD27+) per µl of blood. *P<0.05, **P<0.01, ***P<0.001 using a post hoc Dunn’s test. In (B) and (E) the Kruskal–Wallis value is shown. MMF, mycofenolate mofetil.
SCC Development During Study
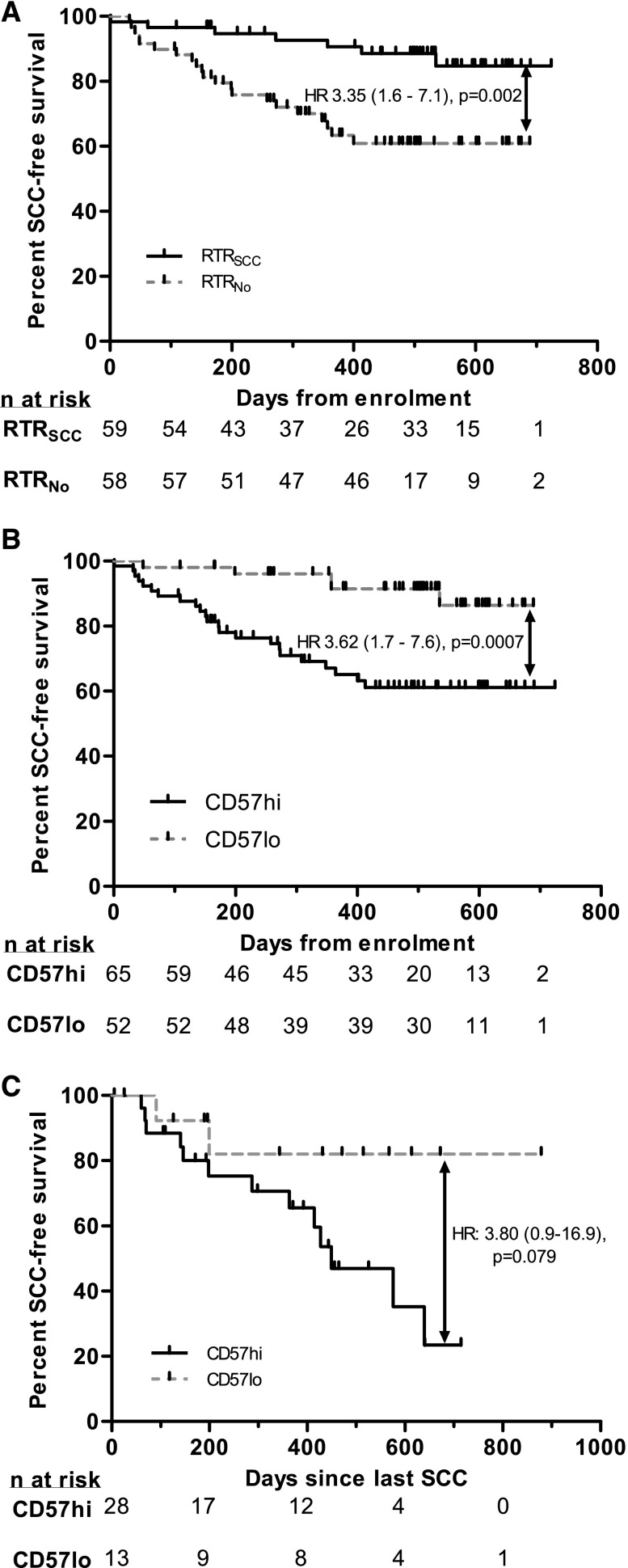
During median (interquartile range (IQR)) follow-up of 522 (434–607) days from enrolment, 28 (seven RTRNo) participants developed 57 SCC (Figure 3A). Four participants (two RTRNo) died during the study period (three due to cardiovascular disease, one due to hematologic malignancy) and two RTRNo returned to dialysis. There was no difference in follow-up duration between groups.
Figure 3.
CD57 phenotype predicts SCC development and recurrence. (A) SCC occurrence in all RTRs by history of previous SCC. (B) SCC occurrence in all RTRs by CD57 phenotype. (C) SCC recurrence in all RTRs by time since previous SCC (for those with SCC during study or preceding year). Black ticks indicate censoring. Hazard ratios are reported as univariate Cox models.
We analyzed factors predictive of SCC development during follow-up (Table 3). An increasing duration of dialysis before transplantation, increasing age (at enrolment and at first transplant), a history of prestudy SCC and increasing clinical risk scores (for all three scores assessed) were associated with SCC development on univariate analysis. Immunologically, an increased number of γδ T cells (Table 4) and CD57hi phenotype were associated with subsequent SCC development (Figure 3B, Table 4).
Table 3.
Predictive value of clinical risk scores for SCC development during study
| Variable | Univariate | Without age component | ||
|---|---|---|---|---|
| HR (95% CI) | P Value | HR (95% CI) | P Value | |
| Harden risk score | 1.41 (1.10 to 1.81) | 0.01 | 1.24 (0.78 to 1.97) | 0.36 |
| Urwin risk score | 1.42 (1.10 to 1.83) | 0.01 | 1.29 (0.94 to 1.76) | 0.12 |
| Harwood risk score | 1.56 (1.10 to 2.22) | 0.01 | NA | NA |
Variables are expressed as a HR per unit increase in score, calculated by Cox modeling. The univariate analysis was then repeated using the risk score without the age component. It was not possible to do this for the Harwood score due to the nature of the score.
HR, hazard ratio; 9% CI, 95% confidence interval; NA, not applicable.
Table 4.
Predictive value of clinical and immunologic factors for SCC development during study
| Variable | Univariate | Multivariate | ||
|---|---|---|---|---|
| HR (95% CI) | P Value | HR (95% CI) | P Value | |
| Age at enrolment (years) | 1.07 (1.03 to 1.11) | <0.001 | 1.03 (0.99 to 1.08) | 0.14 |
| Male gender | 1.77 (0.72 to 4.37) | 0.22 | ||
| Age at first transplant (years) | 1.04 (1.01 to 1.07) | 0.01 | ||
| Received dialysis pre-transplant | 2.47 (0.75 to 8.18) | 0.14 | ||
| Duration of dialysis pre-transplant (months) | 1.01 (1.00 to 1.01) | 0.04 | 1.00 (1.00 to 1.01) | 0.35 |
| Total immunosuppression duration (months) | 1.00 (1.00 to 1.00) | 0.91 | ||
| Number of HLA mismatches | 1.06 (0.83 to 1.37) | 0.64 | ||
| Estimated GFR at enrolment (ml/min per 1.73 m2) | 1.00 (0.98 to 1.02) | 0.71 | ||
| Cytomegalovirus seropositivity | 2.58 (0.98 to 6.78) | 0.06 | ||
| Positive smoking history | 1.21 (0.57 to 2.59) | 0.62 | ||
| History of previous SCC | 3.66 (1.55 to 8.64) | 0.01 | 3.25 (1.29 to 8.17) | 0.01 |
| History of previous non-NMSC malignancy | 1.04 (0.36 to 2.99) | 0.94 | ||
| Malignancy in first-degree relative | 0.57 (0.26 to 1.26) | 0.17 | ||
| History of chronic UV exposure | 1.93 (0.85 to 4.39) | 0.12 | ||
| Increasing Fitzpatrick skin type (no units) | 0.81 (0.51 to 1.28) | 0.36 | ||
| Non brown eye color | 1.06 (0.47 to 2.40) | 0.89 | ||
| Number of lymphocytes (cell/µl) | 1.00 (0.999 to 1.001) | 0.88 | ||
| Number of CD3+ cells (cell/µl) | 1.00 (0.999 to 1.001) | 0.94 | ||
| Number of CD4+ T cells (cell/µl) | 1.00 (0.998 to 1.000) | 0.20 | ||
| Number of CD8+ T cells (cell/µl) | 1.00 (0.999 to 1.002) | 0.40 | ||
| Number of Treg (cell/µl) | 0.97 (0.924 to 1.007) | 0.10 | ||
| Number of γδ T cells (cell/µl) | 1.01 (1.001 to 1.009) | 0.01 | 1.01 (1.001 to 1.010) | 0.02 |
| Number of CD57+ CD8+ T cells (cell/µl) | 1.00 (1.000 to 1.002) | 0.14 | ||
| >50% CD57+ CD8+ T cells | 4.60 (1.745 to 12.11) | 0.01 | 2.85 (1.02 to 7.95) | 0.05 |
Variables are expressed as a HR, per unit increase for continuous variables (units in parentheses where appropriate). For multivariate modeling >50% CD57+ CD8+ T cells was adjusted for variables significant on univariate analysis (all adjusted values are given). Age at enrolment and age at first transplant correlated strongly (r=0.74) and so only age at enrolment was utilized in the multivariate analysis, as the stronger predictor.
As all three clinical risk scores utilize age,2,3,11 we assessed whether these scores had predictive value above that offered by age alone. When all three were recalculated without including age at first transplant, no score provided predictive value in this cohort, which was also confirmed using receiver operator characteristic (ROC) curves (Supplemental Figure 1, Table 3).
When the number of γδ T cells and CD57hi phenotype were adjusted for age at enrolment, previous SCC and duration of pretransplant dialysis, they remained independently predictive of SCC development (Table 4). Age at enrolment lost predictive value when adjusted for the other covariates. Replacing age at sampling with age at first transplant did not significantly change the results of these analyses (Supplemental Table 2). ROC curves were generated for the number of γδ T cells and percentage of CD57-expressing CD8+ T cells – the c-statistics were similar, but greatest for the percentage of CD57-expressing CD8+ T cells, with the cut-off of 50% close to peak sensitivity and specificity for detecting SCC during follow-up (Supplemental Figure 1).
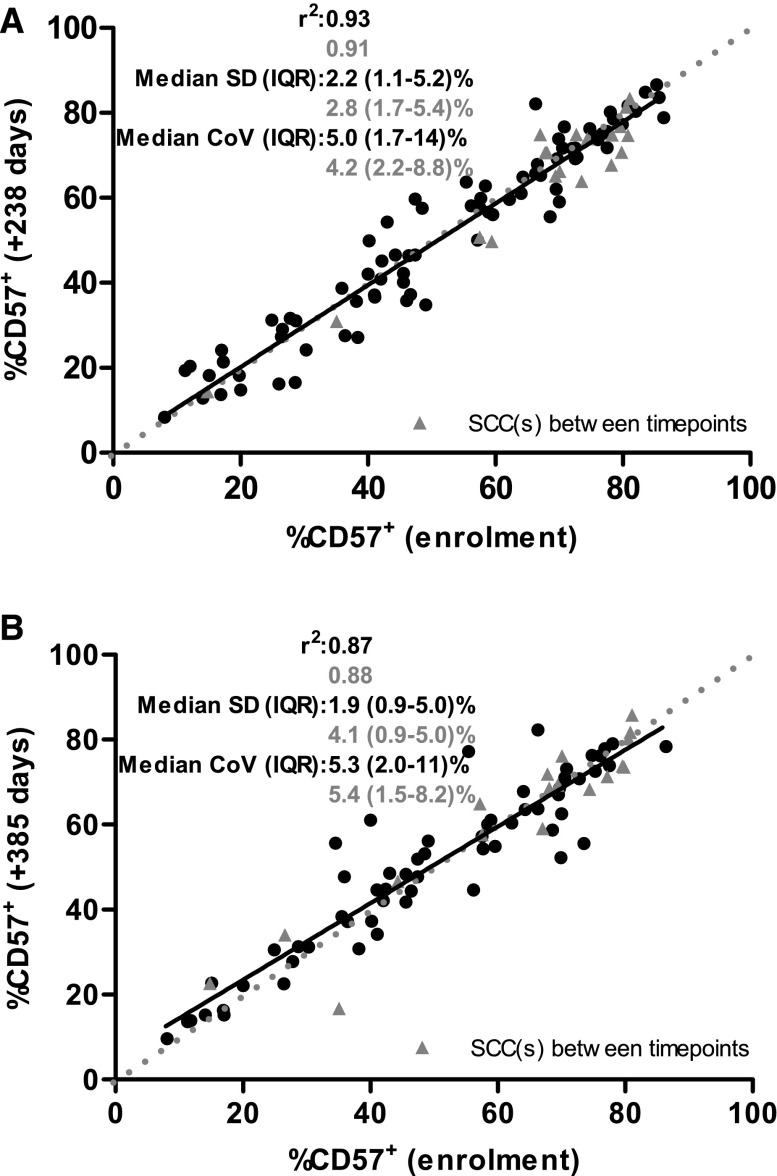
In order to assess the stability of CD57 expression, we retested participants a median (IQR) of 238 (189–252) (n=99) and 385 (364–427) days (n=76) postenrolment. The median (IQR) standard deviation from baseline was 2.2 (1.2–5.1%) and 2.1 (0.9–4.9%), respectively and did not differ when stratified by SCC development (Figure 4). These results suggest that this marker is stable over a 1-year period and with SCC development.
Figure 4.
CD57 expression upon CD8+ T cells is stable with time and SCC development. Correlation of percentage of CD57-expressing CD8+ T cells between enrolment and 238 days (A) and 385 days (B) later. Points in grey indicate RTRs who developed SCC between sampling. Broken line indicates line of perfect correlation, while solid line indicates line of best fit for whole cohort. Coefficient of determination (r2), standard deviation (SD) and coefficient of variation (CoV) are provided stratified by those who developed SCC between sampling (grey triangles) and those who remained SCC-free (black circles).
We next explored disease behavior. We analyzed time between SCC in those who developed SCC during the study or the preceding year, where enrolment CD57 phenotype likely represented that at the time of SCC (n=41). CD57hi RTRs with recent SCC trended towards earlier recurrence, such that only 25% of CD57hi RTRs were free of SCC recurrence, whereas in CD57lo 80% remained SCC-free, at 600 days (Figure 3C). There was no such trend for either age variable or all three risk scores (data not shown). This equated to a positive predictive value of 0.46 and a negative predictive value of 0.85 for further SCC development (Supplemental Table 4). We attribute the failure to reach significance to the small number in the CD57lo arm.
Determinants of CD57 Expression on CD8+ T Cells
Finally, we asked what clinical factors might influence CD57 phenotype. Age and cytomegalovirus seropositivity were independently predictive of a high proportion of CD57-expressing CD8+ T cells (Supplemental Table 1). In support of this, the cytomegalovirus IgG titer showed a weak but significant correlation with the percentage of CD57-expressing CD8+ T cells in those who were cytomegalovirus seropositive (r2 0.17, P<0.001). Cytomegalovirus seropositivity significantly increased the proportion of CD57-expressing TEMRA cells (Figure 1B). Given these findings, we assessed cytomegalovirus viral load on a subset of stable seropositive participants with and without a history of SCC (n=27) during study follow-up: the viral load was below the limit of reliable detection in all.
Discussion
This is the first study to compare clinical and immunologic markers in predicting SCC development in long-term, high-risk RTRs.2 We demonstrate that previously identified immunologic predictive markers, such as NK cell and B cell populations, are significantly affected by azathioprine use.13,22 We found an increased proportion of CD57-expressing CD8+ T cells provided a summary measure for immunosenescence, and was the strongest available predictor of future SCC in this cohort, identifying a subset at nearly threefold increased risk of subsequent SCC, and who may develop earlier recurrent disease, with a greater predictive value than most traditional clinical risk factors. This marker is stable with time and with the development of SCC. For the first time, we report that the differentiation and immunosenescent phenotype may be affected by steroid usage; however, this would potentially act to strengthen the association between SCC development and immunosenescence, as it would be expected that the increased immunosuppression with steroid use (those on steroids were significantly more likely to be on triple immunosuppressive therapy, data not shown) would increase the risk of SCC development.
The effect of azathioprine on NK cells has been previously documented and is reversible upon cessation; given the NK cell-sparing effect of mycophenolate, this may provide a rationale to switch RTRs with SCC from azathioprine to this agent.22
The effect on B cells has only been described recently in a small study, and we validate this finding in a much larger cohort.23 We hypothesize that this effect is due to the inhibition of purine synthesis in bone marrow lymphoblasts.24 This effect is particularly relevant given the alteration in B cell parameters described in immunosuppression-free RTRs with stable graft function (operational tolerance) compared with those still on immunosuppression.25,26
Age at enrolment and age at first transplant were no longer predictive of SCC development when adjusted for CD57hi phenotype, and this may indicate that this previously described risk factor may refer to increasing immune senescence. The possibility of index event bias cannot be ruled out, given that increasing age is a risk factor for SCC and a contributor to CD57 expression; however, on correction for age CD57 expression remained a significant predictor of SCC development and CD57hi RTRs tended to predict earlier SCC recurrence, while age did not.
CD57 expression on T cells represents a marker of terminal differentiation and senescence, in keeping with its predominant expression upon TEMRA cells, with impaired proliferation and reduced production of effector cytokines.17,18 Given the correlation with CD57 expression on CD4+ T cells, this may be indicative of overall T cell senescence. Ex vivo CD8+CD28- and CD8+CD57+ cells with suppressive properties have previously been described in healthy human cohorts27 and in the setting of malignancy28,29 and HIV30 and we cannot exclude a potential suppressive role here.
In nontransplant populations an increased proportion of CD57+ senescent T cells in blood and tumors are associated with a poorer prognosis in various established carcinomata.31–33 In addition, we found the accumulation of CD57+ cells was associated with the loss of both CD4+ and CD8+ TCm, which may provide superior long-lasting antitumor and antiviral immunity.34,35 This may synergize with calcineurin inhibition, which further impairs antitumor responses, and azathioprine, which reduces NK-mediated immune responses. A reduced CD8/FoxP3 ratio within SCC from transplant recipients compared with nontransplant recipients has been demonstrated previously, due to a reduced number of CD8+ T cells rather than an increase in FoxP3+ T cells; our findings may explain this phenomenon.12,36,37
Chronic antigenic stimulation, such as viral infection or autoimmune disease, is associated with an accumulation of senescent and terminally differentiated T cells.38–42 We demonstrate that CD57 expression in cytomegalovirus seropositive RTRs also directly correlates with the titer of anticytomegalovirus IgG. Excess immunosuppression may lead to repeated episodes of subclinical viral reactivation and inflammation over time, leading to the accumulation of oligoclonally expanded senescent T cells that effectively reduces the T cell antigen repertoire.19,43 We did not detect subclinical cytomegalovirus viral load in peripheral blood, but this does not rule out viral load below the limit of detection, viral activity in peripheral tissues, or intermittent reactivation that may be missed by cross-sectional sampling. Cytomegalovirus infection is associated with impaired response to simultaneous Epstein–Barr virus infection.19 Given the postulated carcinogenic role of human papillomavirus in early SCC development, an impaired CD8+ response leading to greater viral load may be particularly significant.44–46
Cytomegalovirus infection has been associated with an increased risk of malignancy in RTRs in the first decade after transplant.41 In contrast, in a small, younger cohort cytomegalovirus exposure was protective against malignancy development in the first 9 years post-transplant through the induction of γδ T cells.47 Hope et al. previously found an increased number of γδ cells to be predictive of SCC development, in agreement with our own data.13 The net effect of cytomegalovirus may depend on malignancy type and the balance between increased numbers of protective γδ T cells and subsequent expansion of differentiated senescent T cells, causing loss of protective immunity to other pathogens. The presence of cytomegalovirus has been reported in SCC biopsies from healthy individuals,48,49 but seropositivity has not previously been associated with increased SCC burden in RTRs.50 Cytomegalovirus seropositivity was not a direct predictor of SCC; other viruses, such as Epstein–Barr and human papillomavirus, may also act to promote terminal differentiation.
We found that three previously published risk scores as well as other risk factors, such as the duration of immunosuppression, did not predict SCC in this population.51 This may relate to the high-risk nature of our cohort in which all have received prolonged immunosuppression. The clinical risk scores examined were developed in populations distinct from this one and may give weight to factors that are useful at a population level, but in a high-risk population such as this do not offer sufficient precision to identify increased SCC risk. Unlike previous studies,12,13 we did not find Treg held prognostic value. This may relate to immunosuppression, as a greater proportion of RTRSCC in this study were on calcineurin inhibitors and at a higher dosage compared with previous studies, which may suppress Treg numbers.
There are limitations of this study to consider. Due to the nature of cohort studies, survivor bias may limit the apparent effect of certain variables. RTRSCC did not have an increased proportion of CD57hi participants, which may be due to increased malignancy-related mortality. Few participants in this study received induction therapy or are using newer immunosuppressive agents, and the effects of these on senescence and SCC risk are unknown. Finally, the study is predominantly in white RTRs and cannot be generalized to other ethnic populations. However, post-transplant SCC is predominantly a disease of Caucasian RTRs and so our cohort reflects those with the greatest disease burden. The effect of the competing risk of death should also be considered. Two deaths occurred after SCC development and the remaining deaths were in CD57hi participants, so any potential effect would be small and would result in a reduction of the association between CD57 and SCC. We did not include keratoacanthoma as an event in this study in order to maximize the homogeneity of the disease outcome. The relationship between keratoacanthoma and SCC is controversial, with keratoacanthoma showing evidence of altered pathophysiology and CD8+ immune response.52
These findings have potential clinical implications. Taken with previous findings, stratification of RTRs by CD57 expression prior to commencement of immunosuppression may allow for tailored education and surveillance with regard to the risk of future malignancy, especially in those triaged as high risk by clinical risk scores. Reduced ciclosporin trough levels are associated with a lower incidence of future cutaneous malignancy.53 Our marker may identify RTRs who would benefit from reduced levels of immunosuppression with lower risk of graft dysfunction. Increasing differentiation in the CD8+ compartment has previously been associated with a reduced incidence of acute rejection postrenal transplant but conversely an increased risk of rejection in liver transplantation.54,55 A recent study suggested a trend towards late allograft dysfunction in RTRs, with an increased proportion of CD57-expressing and TEMRA CD8+ T cells.42 Taken together, these studies and our own suggest that further analysis is required in order to understand their role post-transplant.
In summary, this is the first study that directly associates an increased propensity to cutaneous malignancy and an increased proportion of terminally differentiated or senescent cells in peripheral blood, driven partly by cytomegalovirus infection. We show that CD57 stratification identifies RTRs at a nearly threefold increased risk of subsequent SCC, superior to most standard clinical indicators in this high-risk cohort. This may guide the identification of RTRs who require close dermatologic monitoring, and future studies should focus on the safety of immunosuppression reduction in this population.
Concise Methods
The conduct of the study was approved by the NHS research ethical committee prior to commencement (reference 12/WS/0288) and was conducted according to the principles of the Declaration of Helsinki. Informed consent was given prior to enrolment. The study is reported according to strengthening the reporting of observation studies in epidemiology (STROBE) guidelines. Full methods are available in the Supplemental Information.
Participants
Stable RTRs without recent noncutaneous malignancy were recruited during routine transplant outpatient follow-up between March 2013 and November 2014. All available RTRs with a previous history of SCC were identified and approached, and those who participated were used to recruit a phenotypically similar cohort (in terms of age, gender, and duration of immunosuppression) at a 1:1 ratio. RTRs with a history of pretransplant SCC, or SCC within 1 year of transplant, were excluded. A questionnaire was completed at this time to assess lifetime sun exposure, family history of malignancy and smoking history, and skin type was assessed. Clinical data were collected from medical and transplant records and pathology databases. The eGFR was calculated using the four-variable modification of diet in renal disease equation.56 Chronic UV exposure and clinical risk scores were assessed as described previously.2,3,11 All participants provided written consent prior to enrolment. Sera taken at time of enrolment were tested for IgG to cytomegalovirus by the combined immunoassay-chemiluminescence platform (Diasorin, Dartford, UK); a titer of greater than 14 IU/ml was considered positive. A subset of cytomegalovirus seropositive participants, chosen at random, was tested for cytomegalovirus viral load by PCR during follow-up sampling during the study.
Immune Phenotyping
Blood was taken at trough levels of immunosuppression and stored on ice prior to extraction of PBMCs, which occurred within 4 hours of venepuncture. Briefly, PBMCs were isolated by density-gradient centrifugation and stained using a cocktail of antibodies. Prior to intracellular staining PBMCs were fixed and permeabilized. Data were acquired using a Navios flow cytometer and analyzed using Kaluza version 1.4 (both Beckman Coulter, Wycombe, UK) and FlowJo X (TreeStar, Inc); the gating strategy is illustrated in the Supplemental Information (Supplemental Figure 4). The total lymphocyte count from simultaneous routine hematology laboratory testing was used to calculate absolute cell counts.
Statistical Analyses
The primary outcome was time from study enrolment to first diagnosis of SCC during the study. Secondary outcomes were total number of SCC during follow-up and occurrence of metastatic SCC. All diagnoses of SCC were made on histologic grounds: where diagnostic uncertainty was present, the histologist’s decision was considered final. All diagnoses were made by trained dermatopathologists. If the histologist was unable to provide a favored diagnosis, a nondiagnosis of SCC was presumed. SCC arising within scar tissue or clinically described as recurrence did not count as an event. No patients were lost to follow-up, except for death.
All analyses were performed on Graphpad Prism for Windows 5.03 (Graphpad Software Inc., San Diego, CA) or IBM SPSS Statistics for Windows 20.0 (IBM Corp., New York, NY). Continuous variables are shown as median (IQR) unless specified otherwise, except hazard ratios, which are reported as hazard ratio (95% confidence interval). Categorical variables are reported as number (percentage of group). For continuous variables, comparison between groups was performed using the nonparametric two-tailed Mann–Whitney (two groups) or Kruskal–Wallis (multiple groups) test. For categorical variables the chi-squared test or Fisher’s exact test was used. Where the Kruskal–Wallis test was significant, a subsequent post hoc Dunn’s test was applied. Correlations were tested using Pearson’s test. Survival analyses were performed using Cox regression for SCC development during follow-up and odds ratios were calculated by logistic regression. Multivariate analyses used variables statistically significant on univariate analysis. To investigate the ability of a specific variable to predict SCC, ROC curve analyses were performed. Throughout the study, a P value of less than 0.05 was considered statistically significant.
Disclosures
None.
Supplementary Material
Acknowledgments
The authors would like to thank the staff and patients at the Oxford Transplant Unit for their support of this study, in particular S. Ruse for assisting in coordinating the follow-up of participants.
The authors also thank G. Betts and R. Arroyo-Hornero for their technical assistance, J. van der Net and R. Cipelli for their guidance regarding statistical analysis, and S. Wareing (Oxford University Hospitals Virology Department) for performing cytomegalovirus IgG titer quantification.
The authors acknowledge the support of the National Institute for Health Research, through the Local Clinical Research Network.
These data were presented at the UK Academy of Medical Sciences Spring Meeting 2015 and are published in abstract form in a supplemental edition of The Lancet.
M.J.B. is funded by grants from the Wellcome Trust (Clinical Training Fellowship) and Oxford Hospitals Research Services Committee.
K.J.W. and her group received funding from the following: Wellcome Trust, British Heart Foundation, Medical Research Council, Kidney Research UK, European Union (FP7), OHSRC, the Academy of Medical Sciences and the University of Oxford Medical Research Fund.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
See related editorial, “Cancer in ESRD: Clear on the Epidemiology, Hazy on the Mechanisms,” on pages 1272–1275.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2015030250/-/DCSupplemental.
References
- 1.Zwald FO, Brown M: Skin cancer in solid organ transplant recipients: advances in therapy and management: part I. Epidemiology of skin cancer in solid organ transplant recipients. J Am Acad Dermatol 65: 253–261, quiz 262, 2011 [DOI] [PubMed] [Google Scholar]
- 2.Harwood CA, Mesher D, McGregor JM, Mitchell L, Leedham-Green M, Raftery M, Cerio R, Leigh IM, Sasieni P, Proby CM: A surveillance model for skin cancer in organ transplant recipients: A 22-year prospective study in an ethnically diverse population. Am J Transplant 13: 119–129, 2013 [DOI] [PubMed] [Google Scholar]
- 3.Urwin HR, Jones PW, Harden PN, Ramsay HM, Hawley CM, Nicol DL, Fryer AA: Predicting risk of nonmelanoma skin cancer and premalignant skin lesions in renal transplant recipients. Transplantation 87: 1667–1671, 2009 [DOI] [PubMed] [Google Scholar]
- 4.Buell JF, Hanaway MJ, Thomas M, Alloway RR, Woodle ES: Skin cancer following transplantation: the Israel Penn International Transplant Tumor Registry experience. Transplant Proc 37: 962–963, 2005 [DOI] [PubMed] [Google Scholar]
- 5.Euvrard S, Kanitakis J, Decullier E, Butnaru AC, Lefrançois N, Boissonnat P, Sebbag L, Garnier JL, Pouteil-Noble C, Cahen R, Morelon E, Touraine JL, Claudy A, Chapuis F: Subsequent skin cancers in kidney and heart transplant recipients after the first squamous cell carcinoma. Transplantation 81: 1093–1100, 2006 [DOI] [PubMed] [Google Scholar]
- 6.Wisgerhof HC, Edelbroek JR, de Fijter JW, Haasnoot GW, Claas FH, Willemze R, Bavinck JN: Subsequent squamous- and basal-cell carcinomas in kidney-transplant recipients after the first skin cancer: cumulative incidence and risk factors. Transplantation 89: 1231–1238, 2010 [DOI] [PubMed] [Google Scholar]
- 7.Sheil AG, Disney AP, Mathew TH, Amiss N: De novo malignancy emerges as a major cause of morbidity and late failure in renal transplantation. Transplant Proc 25: 1383–1384, 1993 [PubMed] [Google Scholar]
- 8.Winkelhorst, JT, Brokelman, WJ, Tiggeler, RG, Wobbes, T: Incidence and clinical course of de-novo malignancies in renal allograft recipients. Eur J Surg Oncol, 27: 409-413, 2001 [DOI] [PubMed]
- 9.Martinez JC, Otley CC, Stasko T, Euvrard S, Brown C, Schanbacher CF, Weaver AL; Transplant-Skin Cancer Collaborative : Defining the clinical course of metastatic skin cancer in organ transplant recipients: a multicenter collaborative study. Arch Dermatol 139: 301–306, 2003 [DOI] [PubMed] [Google Scholar]
- 10.Sherston SN, Carroll RP, Harden PN, Wood KJ: Predictors of cancer risk in the long-term solid-organ transplant recipient. Transplantation 97: 605–611, 2014 [DOI] [PubMed] [Google Scholar]
- 11.Harden PN, Fryer AA, Reece S, Smith AG, Ramsay HM: Annual incidence and predicted risk of nonmelanoma skin cancer in renal transplant recipients. Transplant Proc 33: 1302–1304, 2001 [DOI] [PubMed] [Google Scholar]
- 12.Carroll RP, Segundo DS, Hollowood K, Marafioti T, Clark TG, Harden PN, Wood KJ: Immune phenotype predicts risk for posttransplantation squamous cell carcinoma. J Am Soc Nephrol 21: 713–722, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hope CM, Grace BS, Pilkington KR, Coates PT, Bergmann IP, Carroll RP: The immune phenotype may relate to cancer development in kidney transplant recipients. Kidney Int 86: 175–183, 2014 [DOI] [PubMed] [Google Scholar]
- 14.Mahnke YD, Brodie TM, Sallusto F, Roederer M, Lugli E: The who’s who of T-cell differentiation: human memory T-cell subsets. Eur J Immunol 43: 2797–2809, 2013 [DOI] [PubMed] [Google Scholar]
- 15.Hamann D, Baars PA, Rep MH, Hooibrink B, Kerkhof-Garde SR, Klein MR, van Lier RA: Phenotypic and functional separation of memory and effector human CD8+ T cells. J Exp Med 186: 1407–1418, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chattopadhyay PK, Betts MR, Price DA, Gostick E, Horton H, Roederer M, De Rosa SC: The cytolytic enzymes granyzme A, granzyme B, and perforin: expression patterns, cell distribution, and their relationship to cell maturity and bright CD57 expression. J Leukoc Biol 85: 88–97, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Strioga M, Pasukoniene V, Characiejus D: CD8+ CD28- and CD8+ CD57+ T cells and their role in health and disease. Immunology 134: 17–32, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brenchley JM, Karandikar NJ, Betts MR, Ambrozak DR, Hill BJ, Crotty LE, Casazza JP, Kuruppu J, Migueles SA, Connors M, Roederer M, Douek DC, Koup RA: Expression of CD57 defines replicative senescence and antigen-induced apoptotic death of CD8+ T cells. Blood 101: 2711–2720, 2003 [DOI] [PubMed] [Google Scholar]
- 19.Khan N, Hislop A, Gudgeon N, Cobbold M, Khanna R, Nayak L, Rickinson AB, Moss PA: Herpesvirus-specific CD8 T cell immunity in old age: cytomegalovirus impairs the response to a coresident EBV infection. J Immunol 173: 7481–7489, 2004 [DOI] [PubMed] [Google Scholar]
- 20.Goronzy JJ, Fulbright JW, Crowson CS, Poland GA, O’Fallon WM, Weyand CM: Value of immunological markers in predicting responsiveness to influenza vaccination in elderly individuals. J Virol 75: 12182–12187, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wikby A, Månsson IA, Johansson B, Strindhall J, Nilsson SE: The immune risk profile is associated with age and gender: findings from three Swedish population studies of individuals 20–100 years of age. Biogerontology 9: 299–308, 2008 [DOI] [PubMed] [Google Scholar]
- 22.Carroll RP, Hester J, Wood KJ, Harden PN: Conversion to sirolimus in kidney transplant recipients with squamous cell cancer and changes in immune phenotype. Nephrol Dial Transplant 28: 462–465, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moreso F, Torres IB, Martínez-Gallo M, Benlloch S, Cantarell C, Perelló M, Jimeno J, Pujol-Borrell R, Seron D: Gene expression signature of tolerance and lymphocyte subsets in stable renal transplants: results of a cross-sectional study. Transpl Immunol 31: 11–16, 2014 [DOI] [PubMed] [Google Scholar]
- 24.Kazmers IS, Daddona PE, Dalke AP, Kelley WN: Effect of immunosuppressive agents on human T and B lymphoblasts. Biochem Pharmacol 32: 805–810, 1983 [DOI] [PubMed] [Google Scholar]
- 25.Newell KA, Asare A, Kirk AD, Gisler TD, Bourcier K, Suthanthiran M, Burlingham WJ, Marks WH, Sanz I, Lechler RI, Hernandez-Fuentes MP, Turka LA, Seyfert-Margolis VL; Immune Tolerance Network ST507 Study Group : Identification of a B cell signature associated with renal transplant tolerance in humans. J Clin Invest 120: 1836–1847, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sagoo P, Perucha E, Sawitzki B, Tomiuk S, Stephens DA, Miqueu P, Chapman S, Craciun L, Sergeant R, Brouard S, Rovis F, Jimenez E, Ballow A, Giral M, Rebollo-Mesa I, Le Moine A, Braudeau C, Hilton R, Gerstmayer B, Bourcier K, Sharif A, Krajewska M, Lord GM, Roberts I, Goldman M, Wood KJ, Newell K, Seyfert-Margolis V, Warrens AN, Janssen U, Volk HD, Soulillou JP, Hernandez-Fuentes MP, Lechler RI: Development of a cross-platform biomarker signature to detect renal transplant tolerance in humans. J Clin Invest 120: 1848–1861, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang EC, Lehner PJ, Graham S, Borysiewicz LK: CD8high (CD57+) T cells in normal, healthy individuals specifically suppress the generation of cytotoxic T lymphocytes to Epstein–Barr virus-transformed B cell lines. Eur J Immunol 24: 2903–2909, 1994 [DOI] [PubMed] [Google Scholar]
- 28.Filaci G, Fenoglio D, Fravega M, Ansaldo G, Borgonovo G, Traverso P, Villaggio B, Ferrera A, Kunkl A, Rizzi M, Ferrera F, Balestra P, Ghio M, Contini P, Setti M, Olive D, Azzarone B, Carmignani G, Ravetti JL, Torre G, Indiveri F: CD8+ CD28- T regulatory lymphocytes inhibiting T cell proliferative and cytotoxic functions infiltrate human cancers. J Immunol 179: 4323–4334, 2007 [DOI] [PubMed] [Google Scholar]
- 29.Frassanito MA, Silvestris F, Cafforio P, Dammacco F: CD8+/CD57 cells and apoptosis suppress T-cell functions in multiple myeloma. Br J Haematol 100: 469–477, 1998 [DOI] [PubMed] [Google Scholar]
- 30.Sadat-Sowti B, Debré P, Mollet L, Quint L, Hadida F, Leblond V, Bismuth G, Autran B: An inhibitor of cytotoxic functions produced by CD8+CD57+ T lymphocytes from patients suffering from AIDS and immunosuppressed bone marrow recipients. Eur J Immunol 24: 2882–2888, 1994 [DOI] [PubMed] [Google Scholar]
- 31.Akagi J, Baba H: Prognostic value of CD57(+) T lymphocytes in the peripheral blood of patients with advanced gastric cancer. Int J Clin Oncol 13: 528–535, 2008 [DOI] [PubMed] [Google Scholar]
- 32.Characiejus D, Pasukoniene V, Jonusauskaite R, Azlauskaite N, Aleknavicius E, Mauricas M, Otter WD: Peripheral blood CD8highCD57+ lymphocyte levels may predict outcome in melanoma patients treated with adjuvant interferon-alpha. Anticancer Res 28[2B]: 1139–1142, 2008 [PubMed] [Google Scholar]
- 33.Characiejus D, Pasukoniene V, Kazlauskaite N, Valuckas KP, Petraitis T, Mauricas M, Den Otter W: Predictive value of CD8highCD57+ lymphocyte subset in interferon therapy of patients with renal cell carcinoma. Anticancer Res 22[6B]: 3679–3683, 2002 [PubMed] [Google Scholar]
- 34.Klebanoff CA, Gattinoni L, Torabi-Parizi P, Kerstann K, Cardones AR, Finkelstein SE, Palmer DC, Antony PA, Hwang ST, Rosenberg SA, Waldmann TA, Restifo NP: Central memory self/tumor-reactive CD8+ T cells confer superior antitumor immunity compared with effector memory T cells. Proc Natl Acad Sci U S A 102: 9571–9576, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Adams AB, Williams MA, Jones TR, Shirasugi N, Durham MM, Kaech SM, Wherry EJ, Onami T, Lanier JG, Kokko KE, Pearson TC, Ahmed R, Larsen CP: Heterologous immunity provides a potent barrier to transplantation tolerance. J Clin Invest 111: 1887–1895, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mühleisen B, Petrov I, Gächter T, Kurrer M, Schärer L, Dummer R, French LE, Hofbauer GF: Progression of cutaneous squamous cell carcinoma in immunosuppressed patients is associated with reduced CD123+ and FOXP3+ cells in the perineoplastic inflammatory infiltrate. Histopathology 55: 67–76, 2009 [DOI] [PubMed] [Google Scholar]
- 37.Zhang S, Fujita H, Mitsui H, Yanofsky VR, Fuentes-Duculan J, Pettersen JS, Suárez-Fariñas M, Gonzalez J, Wang CQ, Krueger JG, Felsen D, Carucci JA: Increased Tc22 and Treg/CD8 ratio contribute to aggressive growth of transplant associated squamous cell carcinoma. PLoS One 8: e62154, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Focosi D, Bestagno M, Burrone O, Petrini M: CD57+ T lymphocytes and functional immune deficiency. J Leukoc Biol 87: 107–116, 2010 [DOI] [PubMed] [Google Scholar]
- 39.Hadrup SR, Strindhall J, Køllgaard T, Seremet T, Johansson B, Pawelec G, thor Straten P, Wikby A: Longitudinal studies of clonally expanded CD8 T cells reveal a repertoire shrinkage predicting mortality and an increased number of dysfunctional cytomegalovirus-specific T cells in the very elderly. J Immunol 176: 2645–2653, 2006 [DOI] [PubMed] [Google Scholar]
- 40.Wang EC, Lawson TM, Vedhara K, Moss PA, Lehner PJ, Borysiewicz LK: CD8high+ (CD57+) T cells in patients with rheumatoid arthritis. Arthritis Rheum 40: 237–248, 1997 [DOI] [PubMed] [Google Scholar]
- 41.Courivaud C, Bamoulid J, Gaugler B, Roubiou C, Arregui C, Chalopin JM, Borg C, Tiberghien P, Woronoff-Lemsi MC, Saas P, Ducloux D: Cytomegalovirus exposure, immune exhaustion and cancer occurrence in renal transplant recipients. Transpl Int 25: 948–955, 2012 [DOI] [PubMed] [Google Scholar]
- 42.Yap M, Boeffard F, Clave E, Pallier A, Danger R, Giral M, Dantal J, Foucher Y, Guillot-Gueguen C, Toubert A, Soulillou JP, Brouard S, Degauque N: Expansion of highly differentiated cytotoxic terminally differentiated effector memory CD8+ T cells in a subset of clinically stable kidney transplant recipients: a potential marker for late graft dysfunction. J Am Soc Nephrol 25: 1856–1868, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Miqueu P, Degauque N, Guillet M, Giral M, Ruiz C, Pallier A, Braudeau C, Roussey-Kesler G, Ashton-Chess J, Doré JC, Thervet E, Legendre C, Hernandez-Fuentes MP, Warrens AN, Goldman M, Volk HD, Janssen U, Wood KJ, Lechler RI, Bertrand D, Sébille V, Soulillou JP, Brouard S: Analysis of the peripheral T-cell repertoire in kidney transplant patients. Eur J Immunol 40: 3280–3290, 2010 [DOI] [PubMed] [Google Scholar]
- 44.McLaughlin-Drubin ME, Meyers J, Munger K: Cancer associated human papillomaviruses. Curr Opin Virol 2: 459–466, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Weissenborn SJ, Nindl I, Purdie K, Harwood C, Proby C, Breuer J, Majewski S, Pfister H, Wieland U: Human papillomavirus-DNA loads in actinic keratoses exceed those in non-melanoma skin cancers. J Invest Dermatol 125: 93–97, 2005 [DOI] [PubMed] [Google Scholar]
- 46.Neale, RE, Weissenborn, S, Abeni, D, Bavinck, JN, Euvrard, S, Feltkamp, MC, Green, AC, Harwood, C, de Koning, M, Naldi, L, Nindl, I, Pawlita, M, Proby, C, Quint, WG, Waterboer, T, Wieland, U, Pfister, H: Human papillomavirus load in eyebrow hair follicles and risk of cutaneous squamous cell carcinoma. Cancer Epidemiol Biomarkers Prev, 22: 719-727, 2013 [DOI] [PubMed]
- 47.Couzi L, Levaillant Y, Jamai A, Pitard V, Lassalle R, Martin K, Garrigue I, Hawchar O, Siberchicot F, Moore N, Moreau JF, Dechanet-Merville J, Merville P: Cytomegalovirus-induced gammadelta T cells associate with reduced cancer risk after kidney transplantation. J Am Soc Nephrol 21: 181–188, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zafiropoulos A, Tsentelierou E, Billiri K, Spandidos DA: Human herpes viruses in non-melanoma skin cancers. Cancer Lett 198: 77–81, 2003 [DOI] [PubMed] [Google Scholar]
- 49.Zaravinos A, Kanellou P, Spandidos DA: Viral DNA detection and RAS mutations in actinic keratosis and nonmelanoma skin cancers. Br J Dermatol 162: 325–331, 2010 [DOI] [PubMed] [Google Scholar]
- 50.Ingvar Å, Ekström Smedby K, Lindelöf B, Fernberg P, Bellocco R, Tufveson G, Höglund P, Adami J: No association between infections, HLA type and other transplant-related factors and risk of cutaneous squamous cell carcinoma in solid organ transplant recipients. Acta Derm Venereol 92: 609–614, 2012 [DOI] [PubMed] [Google Scholar]
- 51.Mudigonda T, Levender MM, O’Neill JL, West CE, Pearce DJ, Feldman SR: Incidence, risk factors, and preventative management of skin cancers in organ transplant recipients: a review of single- and multicenter retrospective studies from 2006 to 2010. Dermatol Surg 39: 345–364, 2013 [DOI] [PubMed] [Google Scholar]
- 52.Ko CJ: Keratoacanthoma: facts and controversies. Clin Dermatol 28: 254–261, 2010 [DOI] [PubMed] [Google Scholar]
- 53.Dantal J, Hourmant M, Cantarovich D, Giral M, Blancho G, Dreno B, Soulillou JP: Effect of long-term immunosuppression in kidney-graft recipients on cancer incidence: randomised comparison of two cyclosporin regimens. Lancet 351: 623–628, 1998 [DOI] [PubMed] [Google Scholar]
- 54.Betjes MG, Meijers RW, de Wit EA, Weimar W, Litjens NH: Terminally differentiated CD8+ Temra cells are associated with the risk for acute kidney allograft rejection. Transplantation 94: 63–69, 2012 [DOI] [PubMed] [Google Scholar]
- 55.Donckier V, Craciun L, Miqueu P, Troisi RI, Lucidi V, Rogiers X, Boon N, Degré D, Buggenhout A, Moreno C, Gustot T, Sainz-Barriga M, Bourgeois N, Colle I, Van Vlierberghe H, Amrani M, Remmelink M, Lemmers A, Roelen DL, Claas FH, Reinke P, Sawitzki B, Volk HD, Le Moine A, de Hemptinne B, Goldman M: Expansion of memory-type CD8+ T cells correlates with the failure of early immunosuppression withdrawal after cadaver liver transplantation using high-dose ATG induction and rapamycin. Transplantation 96: 306–315, 2013 [DOI] [PubMed] [Google Scholar]
- 56.Shaffi K, Uhlig K, Perrone RD, Ruthazer R, Rule A, Lieske JC, Navis G, Poggio ED, Inker LA, Levey AS: Performance of creatinine-based GFR estimating equations in solid-organ transplant recipients. Am J Kidney Dis 63: 1007–1018, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.