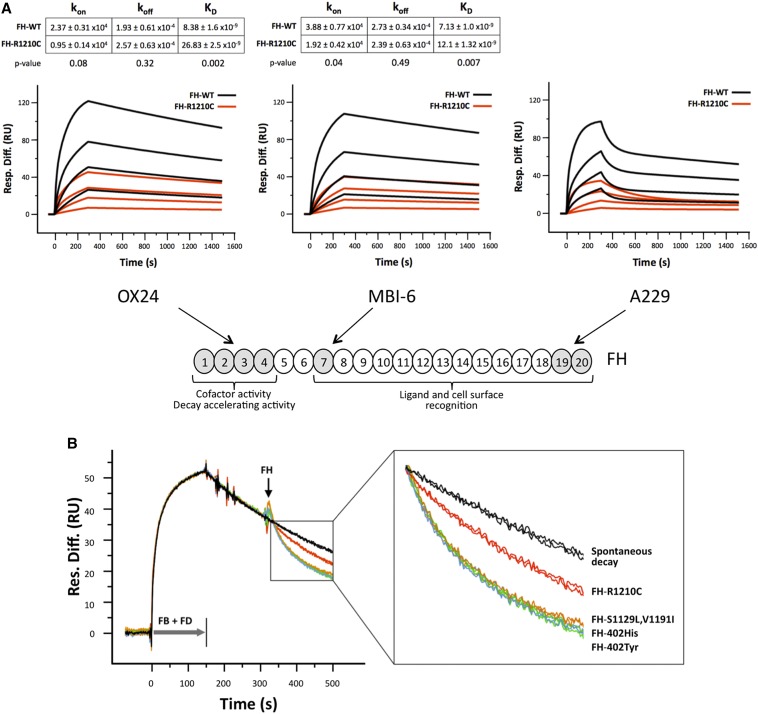
Figure 3.
The R1210C mutation impairs all FH functional domains. (A) SPR analysis showing mAb binding to functional domains of FH variants. Panels depict the binding of purified FH-WT (black) and FH-R1210C (red) (both 402Y) to captured Ox24, MBI-6, and A229 mAbs at increasing concentrations (1 μM, 333 nM, 111 nM, and 37 nM). Sensograms for Ox24 and MBI-6 mAbs show the fitted curves and kinetic constants calculated using a one-to-one interaction model. Values are the means of three independent experiments. In contrast with mAbs OX24 and MBI-6, the binding of FH-WT and FH-R1210C to mAb A229 only fits a heterogeneous ligand model, which is defined by multiple kinetic constants. Note that it requires approximately 4–6 times more FH-R1210C than FH-WT protein to achieve similar binding to all three mAbs. (B) SPR analysis of AP C3 convertase decay-accelerating activity of FH variants. In these experiments, we use a very low density C3b-coated Biacore chip (450 RU) to avoid the possibility that the FH N- and C-terminal regions interact simultaneously with two different C3b molecules on the chip surface; AP C3 convertase is formed by flowing FB (1 µM) and FD (40 nM) for 150 seconds. Spontaneous decay (no FH added) is allowed to proceed for another 150 seconds before flowing FH-WT (402Y and 402H), FH-R1210C, and FH-S1129L-V1191I at 70 nM. Changes in RU during FH injection, compared with the spontaneous decay, represent loss of Bb from the C3 convertase due to FH-mediated decay-accelerating activity. The figure depicts two independent assays for each FH variant. An enlarged view of the boxed part of the sensograms is provided to identify the different FH variants. FB, factor B; FD, factor D; Resp. Diff., response difference; RU, resonance units; SPR, surface plasmon resonance; WT, wild-type.