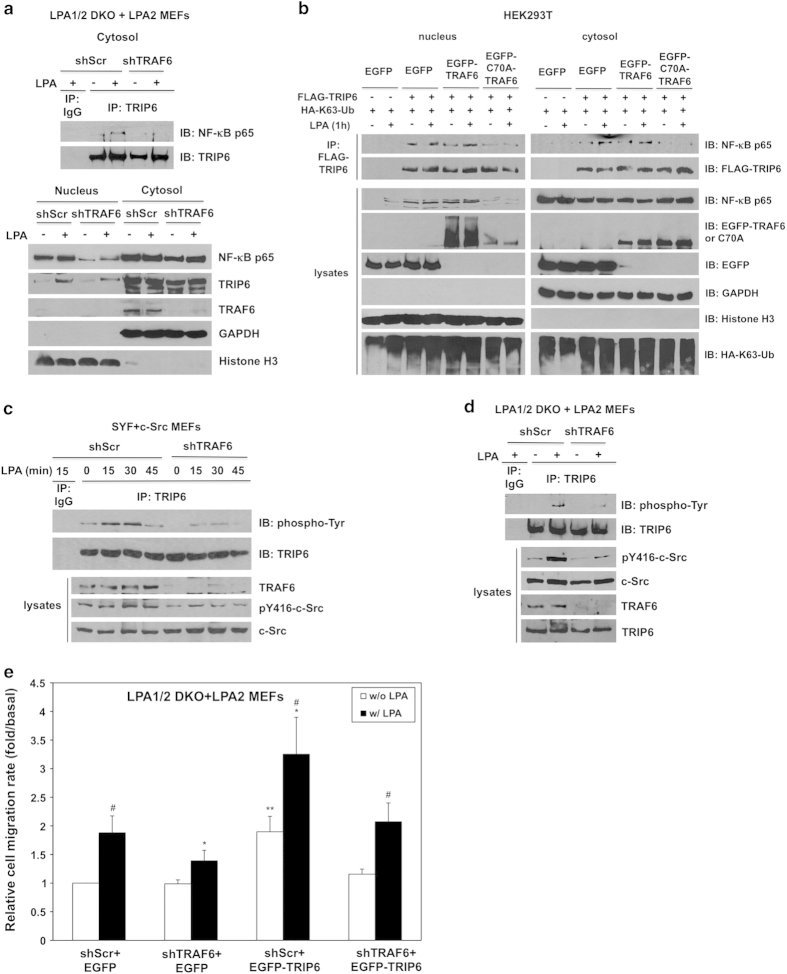
Figure 7.
TRAF6 regulates the functions of TRIP6 in NF-κB p65 binding, c-Src-dependent tyrosine phosphorylation of TRIP6 and LPA2 receptor-mediated cell migration. (a) Knockdown of TRAF6 eliminates LPA-induced association of TRIP6 with NF-κB p65 in the cytosol, and reduces the levels of nuclear NF-κB p65. LPA1/2 DKO MEFs stably expressing LPA2 receptor and either scrambled shRNA or TRAF6 shRNA were starved for 6 h, followed by LPA stimulation for 1 h. Cells were subjected to subcellular fractionation to separate nuclei from the cytosol. Immunoblotting was performed to detect the expression of NF-κB p65, TRIP6 and TRAF6 in each fraction (bottom panel). Histone H3 and GAPDH serve as nuclear and cytosolic markers, respectively. The cytosolic TRIP6 was further immunoprecipitated with anti-TRIP6 mouse monoclonal antibody or control mouse IgG, followed by immunoblotting using anti-NF-κB p65 rabbit antibody to detect the association of TRIP6 with NF-κB p65 in the cytosol (top panel). (b) Overexpression of ligase-defective C70A-TRAF6 eliminates the association of TRIP6 with NF-κB p65. HEK293T cells transiently expressing FLAG-TRIP6, HA-K63-ubiquitin and either EGFP, EGFP-TRAF6 or EGFP-C70A-TRAF6 were starved for 6 h, followed by LPA stimulation for 1 h. Subcellular fractionation was performed in hypotonic buffer to separate nuclei (pellet) from the cytosol (supernatant). FLAG-TRIP6 in each fraction was immunoprecipitated with anti-FLAG M2 mouse monoclonal antibody-conjugated agarose beads, followed by immunoblotting to detect co-immunoprecipitated endogenous NF-κB p65. The immunoblot was reprobed with anti-FLAG antibody to detect precipitated FLAG-TRIP6. The expression of HA-ubiquitin or EGFP fusion proteins in each fraction was detected by immunoblotting using anti-HA or anti-GFP antibody. GAPDH and Histone H3 serve as cytosolic and nuclear markers, respectively. (c and d) Knockdown of TRAF6 attenuates c-Src kinase activity and reduces LPA-stimulated tyrosine phosphorylation of TRIP6. SYF+c-Src MEFs (c) or LPA1/2 DKO+LPA2 MEFs (d) were infected with lentivirus harboring scrambled shRNA or mouse TRAF6-specific shRNA. Cells were starved for 8 h, followed by LPA stimulation for the indicated times. Endogenous TRIP6 was immunoprecipitated with anti-TRIP6 mouse monoclonal antibody, followed by immunoblotting using HRP-conjugated anti-phospho-tyrosine antibody and anti-TRIP6 rabbit antibody, respectively. The bottom panels show the expression of pY416-c-Src, total c-Src, TRAF6 or TRIP6 in the whole-cell lysates. Data shown in (a–d) are representative of two to four independent experiments. (e) Knockdown of TRAF6 reduces LPA-induced cell migration and attenuates the function of TRIP6 in promoting the LPA2 receptor-mediated cell migration. The LPA1/2 DKO MEFs stably expressing FLAG-LPA2 receptor and either scramble shRNA or TRAF6 shRNA were transduced with lentivirus harboring EGFP or EGFP-TRIP6. LPA was added to the lower chamber of transwells, and cells were allowed to migrate for 6 h. Cells that migrated to the bottom filters of the transwells were fixed and stained with crystal violet. The relative migration rate was defined as the fold-increase of migrated cells compared to untreated shScr+EGFP cells. Data shown are the mean±s.e.m. of three independent experiments (*P<0.05 versus treated shScr+EGFP cells; **P<0.05 versus untreated shScr+EGFP cells; #P<0.05 versus untreated cells; Student’s t-test).