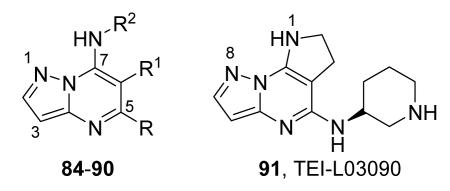
Table 12. Pyrazolopyrimidine derivatives from Teijin Pharma and BioFocus.
| ||||
|---|---|---|---|---|
|
| ||||
| Compd | R | R1 | R2 | IC50a (nM) |
| 84 |
|
H |
|
1300 |
| 85 |
|
Me |
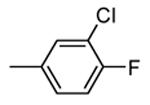
|
400 |
| rac-86 |
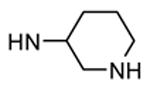
|
H |
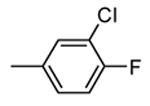
|
40 |
| 87, TEI-I01800b |
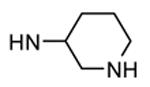
|
Me |
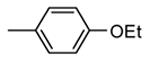
|
130 |
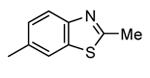
| 88b |
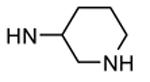
|
Me |
|
57 |
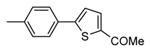
| 89b |
|
Me |
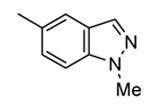
|
76 |
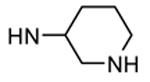
| 90b |
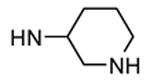
|
Me |
|
54 |
| 91, TEI-L03090 | 4700 | |||
Expressed as the inhibition of MK2 activity toward a peptide substrate.
Compounds 87-90 were in their (S)-configuration.
