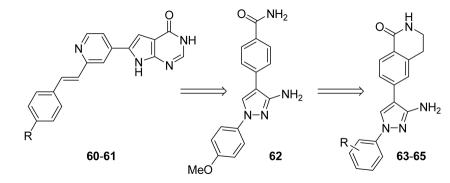
Table 8. Pyrrolopyrimidinones and pyrazoles from Novartis.
| |||
|---|---|---|---|
|
| |||
| Compd | R | IC50a (nM) | EC50b (nM) |
| 60 | F | 200 | 350 |
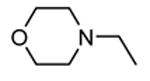
| 61 |
|
51 | 110 |
| 62 | 2000 | ||
| 63 | p-OMe | 84 | |
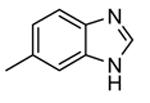
| 64c |
|
82 | 5300 (4100) |
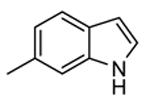
| 65c |
|
61 | 2500 (2000) |
Expressed as the inhibition of MK2 activity toward a peptide substrate.
Expressed as the ability to inhibit TNFα production in hPBMC cells stimulated with both LPS and IFNγ, and to inhibit Hsp27 phosphorylation in anisomycin-stimulated THP-1 cells (in parentheses).
R group is in meta position.
