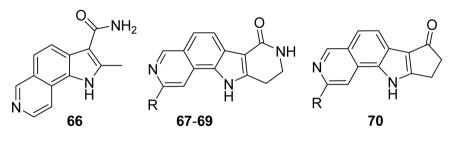
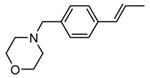
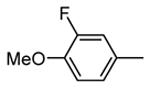
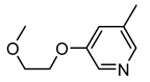
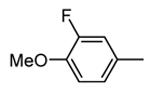
Table 9. Tricyclic and tetracyclic pyrrole derivatives from Novartis.
Expressed as the inhibition of MK2 activity toward a peptide substrate.
Expressed as the ability to inhibit TNFα production in hPBMC cells stimulated with both LPS and IFNγ, and to inhibit Hsp27 phosphorylation in anisomycin-stimulated THP-1 cells (in parentheses).