Figure 3. Quality control of misfolded hERG at the ER .
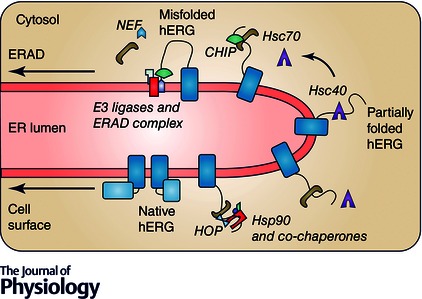
Partially folded hERG is recognized by Hsp40s such as DNAJA1 and DNAJA2. This binding stimulates the hydrolysis of ATP to ADP, initiating Hsp70/Hsc70 substrate binding and Hsp40 dissociation. DNAJA1 assists folding by subsequent binding of Hsp70, Hsp90 and co‐chaperones, resulting in a native form of hERG, which is trafficked to the cell surface. DNAJA2 promotes degradation by initiating CHIP E3 ubiquitin ligase binding. NEFs promote the release of ADP and re‐binding of ATP and release of Hsc70 from the substrate, possibly regulating hERG folding or degradation. Chaperone‐independent E3 ubiquitin ligases and other proteins may be involved in ER associated degradation (ERAD) of hERG.
