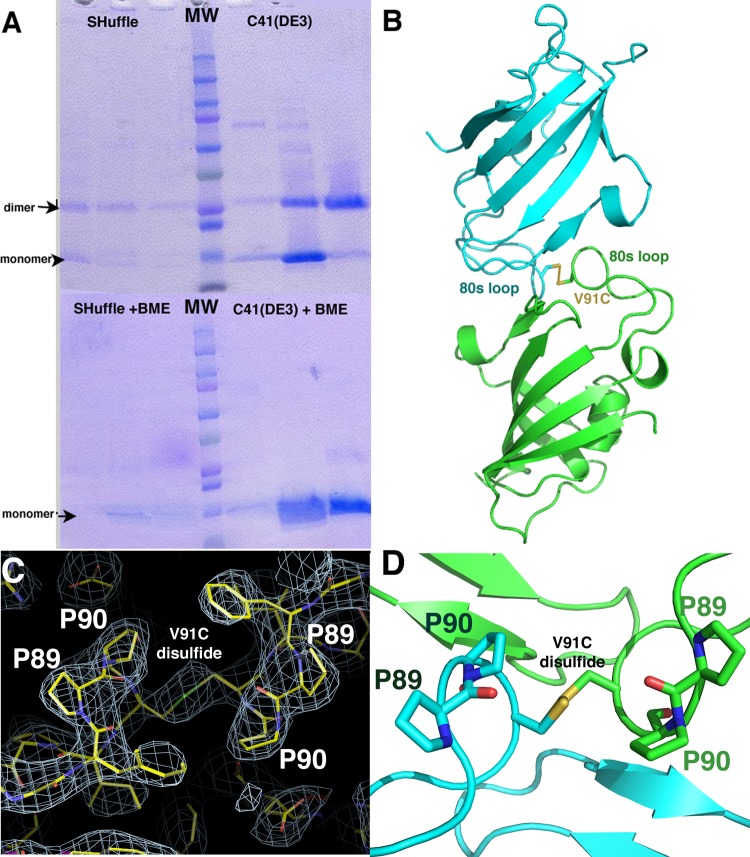
FIG 5 .
A. fumigatus FKBP12(V91C) mutation captures the intermolecular contact. (A) SDS-PAGE purification of FKBP12(V91C) from both SHuffle and C41(DE3) cells. Dimers are evident in the gel run without a reducing agent. Addition of a reducing agent leads to reduction of the disulfide bond and the proteins running as monomers. (B) Crystal structure of A. fumigatus FKBP12(V91C). (C) 2Fo-Fc electron density map contoured at 1 σ showing the region around the V91C disulfide bond and the Pro89-Pro90 bond, which adopts a twisted, trans conformation-like state. (D) Ribbon diagram showing a closeup of the Pro89-Pro90 region with the residues labeled. Note that the green-labeled Pro90 side chains appear distorted.