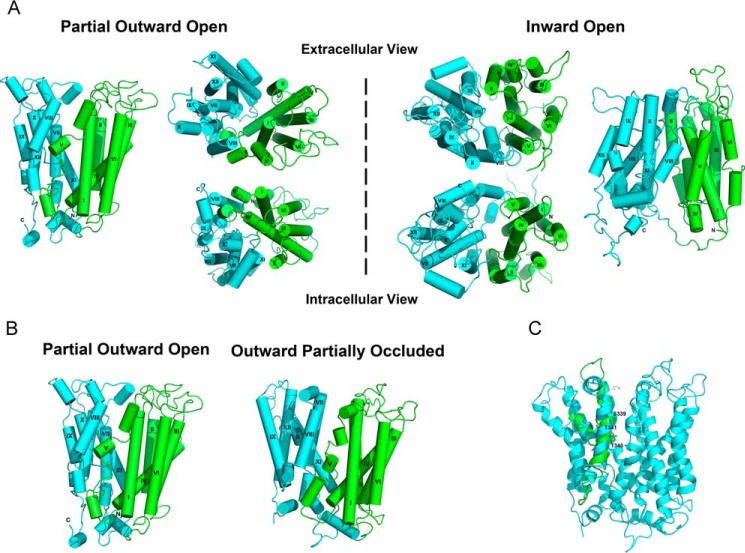
FIGURE 1.
Homology models of MFSD2A. The N- and C-terminal domains are shown in green and cyan, respectively. The helices are labeled with roman numerals. A, overall structure of MFSD2A in the partial outward-open (left of dashed line) and inward-open states (right of dashed line). B, overall structure of MFSD2A in the partial outward-open and outward-partially occluded states. C, rotation of helix VIII in the outward-partially occluded model of MFSD2A to achieve a more likely alignment of the polar side chains of Ser-339, Thr-341, and Thr-343 (labeled in the refined model) toward the protein interior and away from the hydrophobic core of the lipid bilayer. We manually rotated helix VIII in the outward-partially occluded model of MFSD2A. The refined model is shown in cyan and the initial model is shown in green.