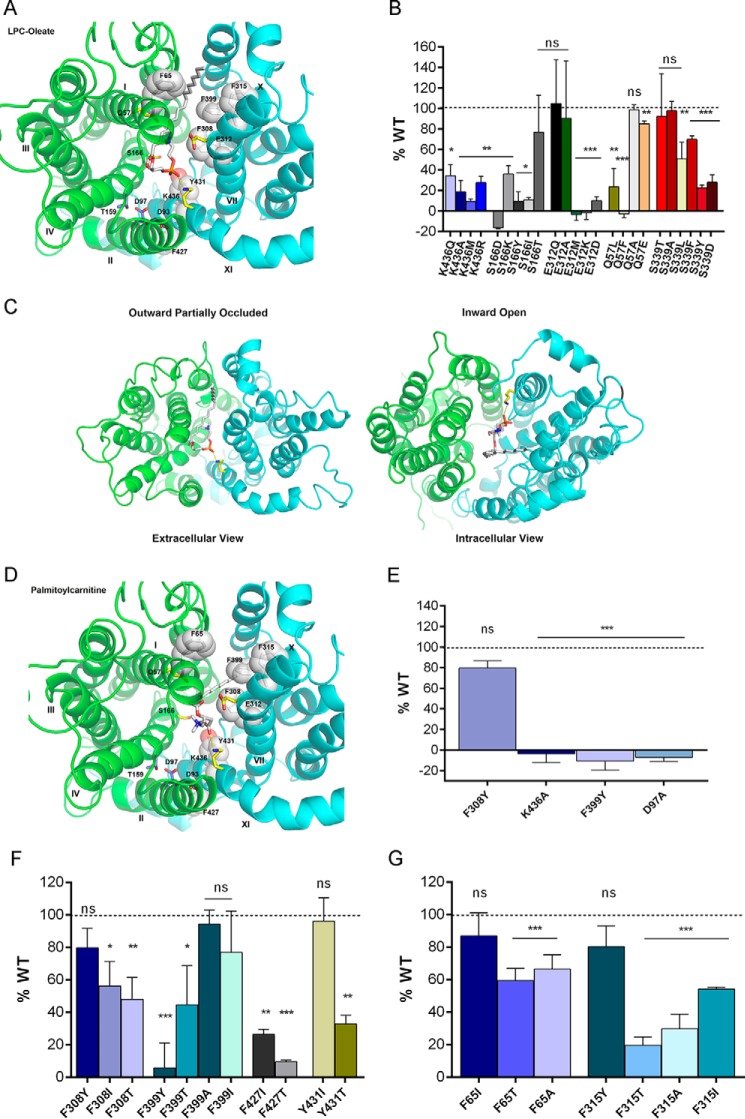
FIGURE 4.
Headgroup binding site and hydrophobic cleft of MFSD2A. A, headgroup binding site (yellow sticks) and hydrophobic cleft (white sticks and spheres) viewed from the extracellular side of the outward-partially occluded model. LPC-oleate is shown docked. B, transport of LPC-[14C]oleate after 30 min by HEK293 cells transiently expressing MFSD2A constructs with indicated mutations in residues proposed to be involved in headgroup binding. C, docking of LPC-oleate in the outward-partially occluded and inward-open models. In both models, the negatively charged phosphate group remains in close proximity to the positively charged side chain of Lys-436. In the outward-partially occluded model, the side chain of Lys-436 is pointing outward, and LPC-oleate lies within the translocation pathway with its fatty acyl chain projecting toward the extracellular surface of MFSD2A. In the inward-open model, the side chain of Lys-436 is pointing downwards, whereas LPC-oleate lies within the translocation pathway with its fatty acyl chain projecting toward the cytoplasmic surface of MFSD2A. D, palmitoylcarnitine shown docked as in A, indicating similar residue interactions as seen with LPC-oleate docking. E, transport of [3H]palmitoylcarnitine after 20 min by HEK293 cells transiently expressing MFSD2A constructs with indicated mutations. F and G, transport of LPC-[14C]oleate after 30 min by HEK293 cells transiently expressing MFSD2A constructs with indicated mutations in residues in the proposed hydrophobic cleft. Experiments in B and E–G were performed twice in triplicate. Data in B and E–G are expressed as the mean ± S.D. *, p < 0.05; **, p < 0.01; ***, p < 0.001; ns, not significant.