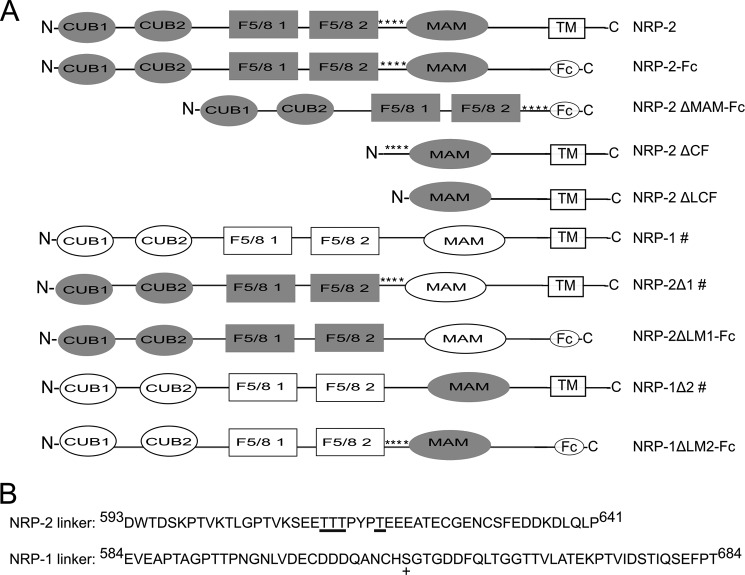
FIGURE 1.
Schematic representation of NRP-1, NRP-2, their MAM and LM chimeras, and domain deletion mutants. A, neuropilin ectodomains are composed of two CUB domains, two F5/8 domains, and a MAM domain. Fc-tagged proteins used in this study lack the transmembrane regions (TM) and cytoplasmic tails of the NRPs, and these sequences are replaced with the human IgG Fc fragment. Both the membrane-associated form with the transmembrane regions and cytoplasmic tail and the soluble Fc form of NRP-2 are shown. Other proteins that are expressed as both membrane-associated and soluble Fc forms are shown as the membrane-associated form and indicated (#). The locations of the threonine residues that carry the polysialylated O-glycans in the NRP-2 linker are indicated (****) in constructs containing these sequences. B, the sequences of the NRP-1 and NRP-2 linker regions between the F5/8-2 and MAM domains are shown. These differ with respect to length and the presence of potential O-glycosylation sites. PolySia is found on the O-glycans occupying four Thr residues in the linker of NRP-2 (these residues are underlined). The serine and threonine residues in the NRP-1 linker that are O-glycosylated are unknown; however, the Ser612 that carries the glycosaminoglycan chain in the NRP-1 linker is indicated (+).