FIGURE 2.
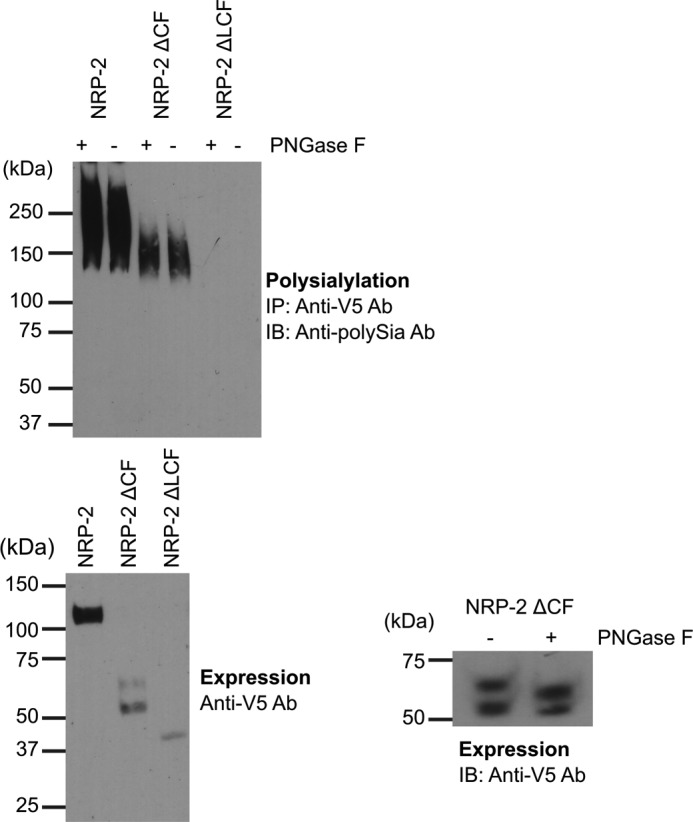
The MAM domain and adjacent linker region are minimally sufficient for NRP-2 polysialylation. Top, NRP proteins were immunoprecipitated from lysates of COS-1 cells transiently expressing ST8SiaIV-Myc and V5-tagged NRP-2 and its domain deletion mutants using an anti-V5 epitope tag antibody. The N-glycans were removed in one-half of each sample by treatment of protein A-Sepharose·antibody·NRP protein complex with PNGase F (+PNGase F), as described under “Experimental Procedures.” Polysialylation of both PNGase F-treated and untreated proteins was assessed by immunoblotting (IB) these immunoprecipitated (IP) proteins with the 12F8 anti-polySia antibody (top). Bottom left, prior to immunoprecipitation, aliquots of cell lysates were heated to 100 °C to remove polySia and immunoblotted with an anti-V5 antibody to evaluate relative expression levels of NRP-2 proteins. Bottom right, the N-glycosylation status of the NRP-2ΔCF doublet was assessed by PNGase F treatment of lysates from expressing cells and immunoblotting with the anti-V5 epitope tag antibody.
