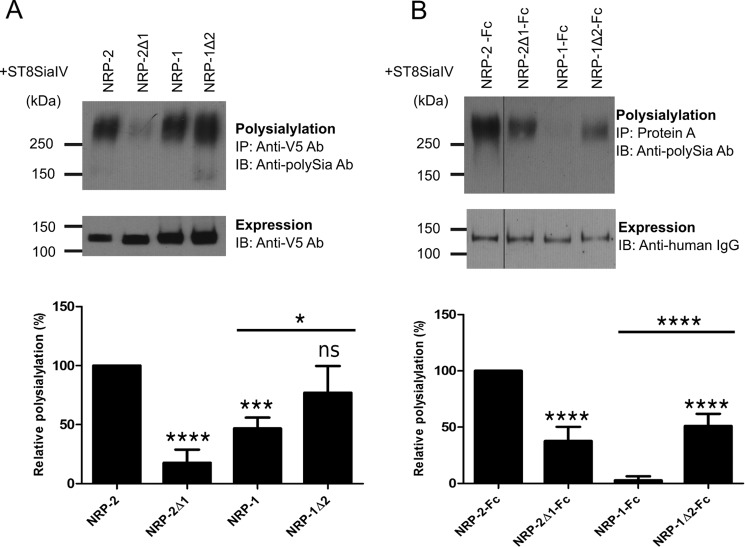
FIGURE 4.
The MAM domain is critical for NRP-2 polysialylation. A, V5-tagged full-length NRP proteins were co-expressed with ST8SiaIV-Myc in COS-1 cells. NRP proteins were immunoprecipitated (IP) from cell lysates using an anti-V5 antibody, and their polysialylation was assessed using the anti-polySia 12F8 antibody (top). Relative expression levels were determined by immunoblotting (IB) a boiled aliquot of each cell lysate using an anti-V5 antibody (bottom). B, soluble, Fc-tagged NRP chimeric proteins were transiently expressed with ST8SiaIV-Myc in COS-1 cells. The proteins were precipitated from the cell medium using protein A-Sepharose beads. Polysialylation was assessed by immunoblotting with the anti-polySia 12F8 antibody (top). Relative protein expression levels were determined by removing the bound protein from half of the protein A-Sepharose beads by boiling and immunoblotting with an HRP-linked anti-human IgG (bottom). The line separating NRP-2-Fc and NRP-2Δ1-Fc reflects the movement of the NRP-2-Fc lane from the same gel for the purposes of direct comparison with NRP-2Δ1-Fc. Quantification of the experiments shown in A and B was performed as described under “Experimental Procedures” with data from 5 and 7 different experiments, respectively, with error bars representing S.D. Statistical analysis was performed using unpaired Student's t tests. *, 0.01 < p < 0.05; ***, 0.0001 < p < 0.001; ****, p ≤ 0.0001; ns, p ≥ 0.05 with respect to wild type NRP-2, which is normalized to 100%. Other comparisons are indicated by a line above the compared bars in the graph.