FIGURE 3.
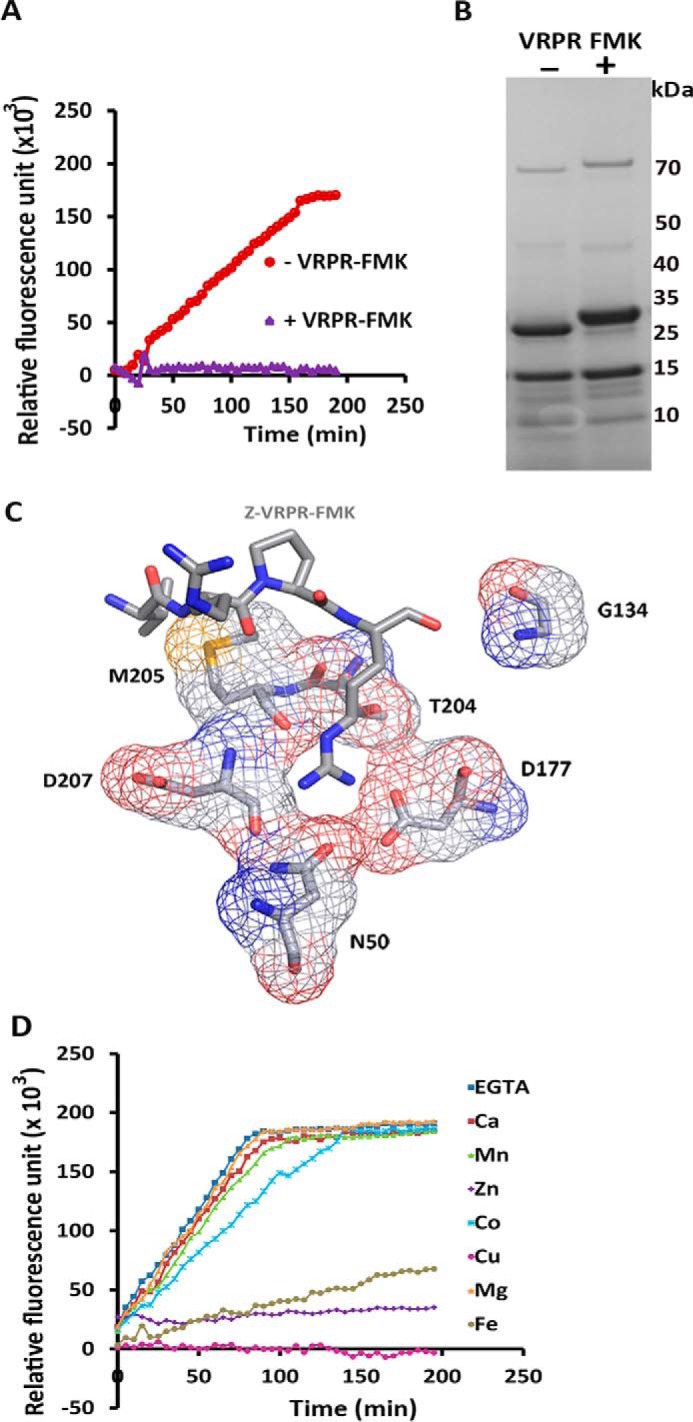
PmC11 binds and is inhibited by Z-VRPR-FMK and does not require Ca2+ for activity. A, PmC11 activity is inhibited by Z-VRPR-FMK. Cleavage of Bz-R-AMC by PmC11 was measured in a fluorometric activity assay with (+, purple) and without (−, red) Z-VRPR-FMK. The relative fluorescence units of AMC released are plotted against time (min) (n = 3; ±S.D.). B, gel-shift assay reveals that Z-VRPR-FMK binds to PmC11. PmC11 was incubated with (+) or without (−) Z-VRPR-FMK and the samples analyzed on a 10% SDS-PAGE gel. A size shift can be observed in the larger processed product of PmC11 (26.1 kDa). C, PmC11 with the Z-VRPR-FMK from the MALT1-paracacaspase (MALT1-P) superimposed. A three-dimensional structural overlay of Z-VRPR-FMK from the MALT1-P complex onto PmC11. The position and orientation of Z-VRPR-FMK was taken from superposition of the PmC11 and MALTI_P structures and indicates the presumed active site of PmC11. Residues surrounding the inhibitor are labeled and represent potentially important binding site residues, labeled in black and shown in an atomic representation. Carbon atoms are shown in gray, nitrogen in blue, and oxygen in red. C, divalent cations do not increase the activity of PmC11. The cleavage of Bz-R-AMC by PmC11 was measured in the presence of the cations Ca2+, Mn2+, Zn2+, Co2+, Cu2+, Mg2+, and Fe3+ with EGTA as a negative control, and relative fluorescence measured against time (min). The addition of cations produced no improvement in activity of PmC11 when compared in the presence of EGTA, suggesting that PmC11 does not require metal ions for proteolytic activity. Furthermore, Cu2+, Fe2+, and Zn2+ appear to inhibit PmC11.
