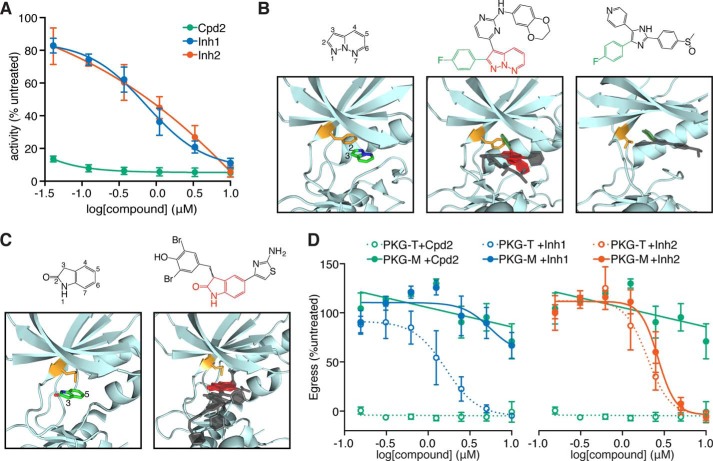
FIGURE 3.
PKG inhibitors display distinct modes of inhibition. A, activity of recombinant PKG in the presence of increasing concentrations of Inh1, Inh2, or the PKG inhibitor Cpd2. B, binding of PP derivatives like Inh1 to protein kinases orients the C2 position toward the gatekeeper residue (orange). Left, the PP scaffold's orientation for a disubstituted PP in complex with CDK2. Middle, the structure of Inh1 is highlighted to indicate the PP scaffold (red) and the C2 fluorophenyl group (green). Two other similar compounds from other kinase structures are superimposed over the CDK2 structure in complex with its inhibitor, all showing a similar positioning of the PP scaffold. Right, human p38 MAPK with a trisubstituted monocyclic heterocycle oriented similarly as the PP scaffold and extending a fluorophenyl group in the direction of the gatekeeper. C, oxindole derivatives bind to protein kinases in a manner that orients their C2 and C5 positions away from the gatekeeper. Left, oxindole scaffold of a derivative in complex with NEK2. Right, the oxindole scaffold of Inh2. Two other similar compounds from other kinase structures have been superimposed on the structure of NEK2 with its inhibitor. D, zaprinast-induced egress of parasites carrying Cpd2-sensitive (PKG-T) or resistant (PKG-M) alleles of PKG, following pretreatment with Inh1, Inh2, or Cpd2. Results shown are mean ± S.E. (error bars) for n = 3 independent experiments.