FIGURE 4.
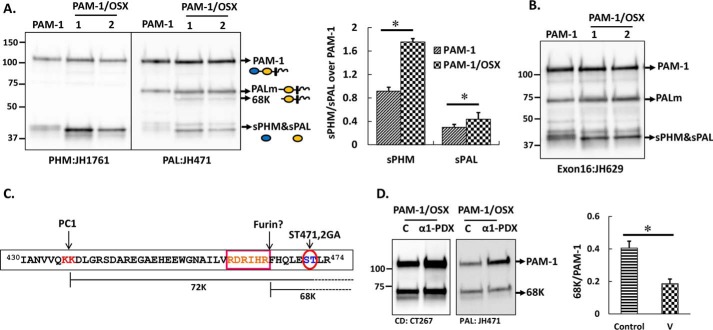
Proteolytic processing of PAM-1 and PAM-1/OSX in AtT-20 cells differs. An AtT-20 line expressing PAM-1 and two lines expressing PAM-1/OSX were compared. A, equal amounts of protein (5 μg) fractionated by SDS-PAGE were visualized using affinity-purified antibodies against PHM or PAL. The major products of PAM-1 cleavage are indicated by arrows. A 68-kDa band was detected with the PAL antibody only in PAM-1/OSX cells. Bar graph showing steady state ratio of sPHM or soluble PAL over PAM-1 in both cell lines was obtained by quantifying blots from five independent experiments (*, p < 0.05). B, when the same samples were probed with an exon 16-specific antibody, the 68-kDa fragment was not recognized. C, schematic identifies a potential furin cleavage site, which could generate 68-kDa PAL. D, PAM-1/OSX cells were infected with adenovirus expressing α1-PDX 48 h before harvest. Cell lysates from control (C) and virus-infected (α1-PDX) cells were prepared in 1% Triton X-100 and fractionated by SDS-PAGE. Western blotting using a CD antibody (left) or a PAL antibody (right) showed a decrease in the amount of 68-kDa PAL. Production of 68-kDa PAL in triplicate samples of control and virus-infected cells from two independent experiments using both antibodies was quantified (*, p < 0.05). v, virus.