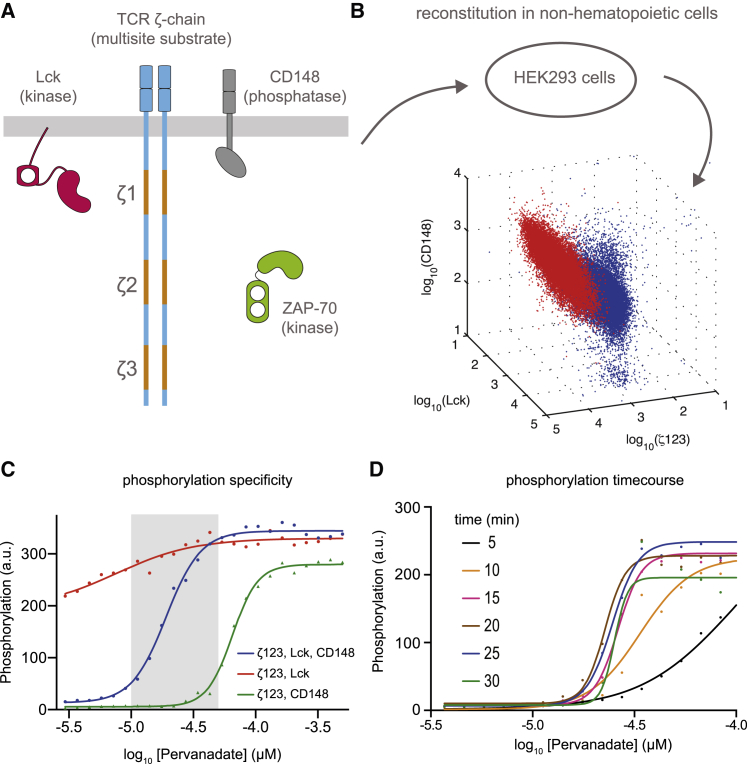
Figure 1.
Cellular reconstitution of multisite phosphorylation of the T cell receptor ζ-chain. (A) Schematic of reconstituted signaling proteins. The substrate is a CD2-TCRζ-chain chimera that contains six phosphorylation sites distributed on three ITAMs (orange) that dimerizes as a result of a disulfide bond in the ζ-chain transmembrane domain (i.e., the substrate is a receptor dimer that contains 12 phosphorylation sites). The substrate is phosphorylated by the membrane-anchored kinase Lck and dephosphorylated by the transmembrane phosphatase CD148. The cytosolic kinase ZAP-70 can bind to phosphorylated ITAMs by SH2 domains. (B) Combinations of these components were transfected into the nonhematopoietic HEK293 cell line and molecular expression was detected with flow cytometry 24 h posttransfection. A k-means clustering algorithm classified the cells as either positive (red, 25%) or negative (blue, 75%) for transfected components (see Fig. S2 for two-dimensional projections). (C) Cells transfected with the indicated components were incubated with increasing concentrations of the tyrosine phosphatase inhibitor pervanadate for 30 min (x axis) before total phosphorylation of the ζ-chain was determined (shaded rectangle highlights the specificity range). (D) Phosphorylation time course for reconstitution of Lck, CD148, and ζ-chain indicates that steady-state phosphorylation is achieved by ∼15–20 min. See Materials and Methods for experimental details. To see this figure in color, go online.