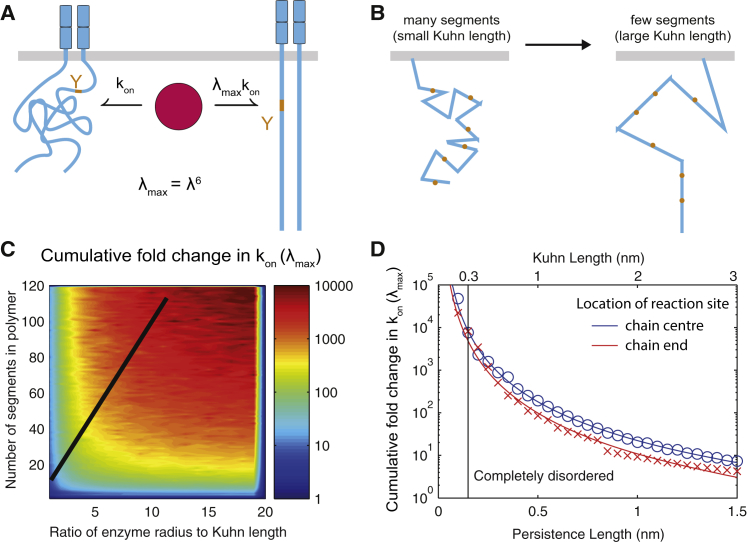
Figure 5.
A phosphorylation-dependent enhancement in the on-rate can arise from a disorder-to-order transition. (A) A polymer model of the ζ-chain predicts that binding of the kinase (or phosphatase) is impeded by entropic disorder (left) and, if phosphorylation imposes local order, this impedance will be reduced when the substrate is phosphorylated with the maximal reduction occurring when the substrate is fully phosphorylated (right). Larger catalytic domains will incur a larger entropic penalty of binding. (B) The degree of disorder is determined by the number of segments whose length is known as the Kuhn length. (C) Heat map showing λmax for different segment numbers (y axis) and for different ratios of the catalytic domain radius to Kuhn length (x axis). (D) Comparison of maximal enhancement over the Kuhn length (or persistence length) when the enzymatic binding site is at the center of the ζ-chain (blue) or at the membrane-distal tyrosine at position 152 (red). A Kuhn length of ∼0.5 or ∼2 amino acids corresponds to λmax ∼ 700. Black line in (C) corresponds to parameter range in (D). See Materials and Methods for computational details. To see this figure in color, go online.