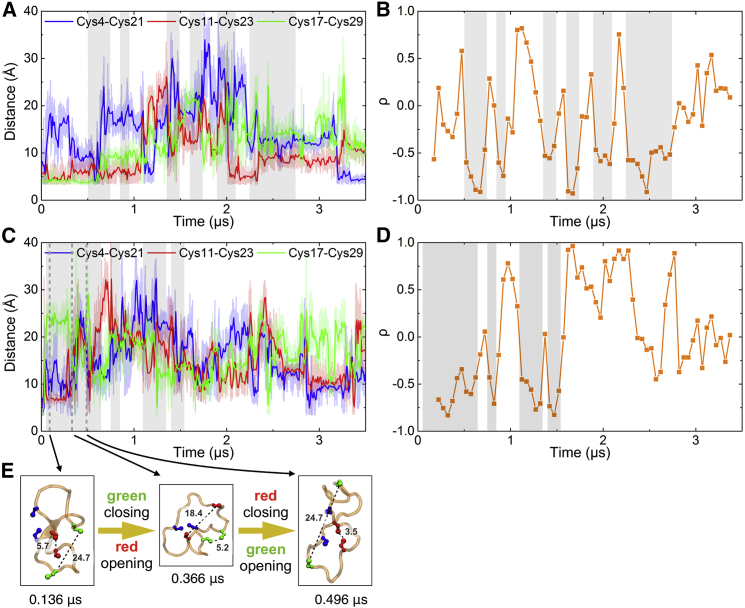
Figure 3.
Sulfur-sulfur distances and correlations monitored in simulations of different conditions. (A) Sulfur-sulfur distances monitored in simulation SS0f(a). Average distances taken over 0.01 μs windows are shown as bold traces; light traces show raw distance data. Gray shading highlights the time when Cys11-Cys23 and Cys17-Cys29 distances are anticorrelated. (B) Pearson correlation coefficient between Cys11-Cys23 and Cys17-Cys29 distances in simulation SS0f(a) from (A). A gliding window of size 0.3 μs and step 0.05 μs is applied to monitor correlation as a function of time. The same gray shading as in (A) cover the time when large negative correlation coefficients are present. (C and D) Same as (A) and (B) for simulation SS0u(a). Other cases are shown in the Supporting Material. At longer times in the simulation, when the peptide is trapped in less nativelike microstates, the anticorrelation disappears (see also Fig. S4). (E) Comparison of structures from simulation SS0u(a) in (C), where an anticorrelation between sulfur-sulfur distances 11–23 (red) and 17–29 (green) is observed. To see this figure in color, go online.