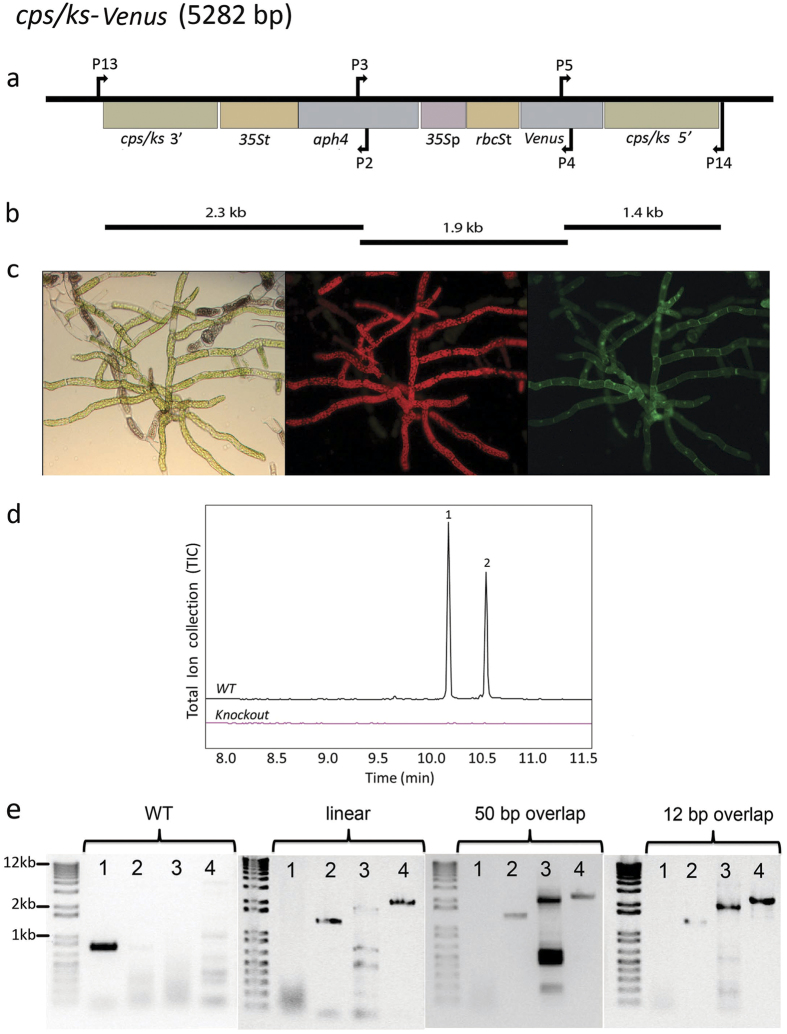
Figure 1. Knockout of the native diterpene synthase CPS/KS gene.
(a) A vector map of the linearized pBK3 vector, including 1000 bp both 5′ and 3′ of the native CPS/KS coding sequence. The 5′ region contains the 1491 bp of the region upstream the ATG for this diterpene synthase gene fused to the gene for the fluorescent protein, Venus. Resistance to hygromycin was conferred by aminoglycoside phosphotransferase 4 coded by aph4 under expression from the 35S promoter. Arrows indicate locations of primer pairs used for PCR genotyping. All genetic maps were created using the software OG Draw (http://ogdraw.mpimp-golm.mpg.de/). (b) Depiction of the three PCR fragments amplified from pBK3, of sizes 2.3, 1.9, and 1.4 kb. (c) Microscopy of Ppcps/ks knockout plants. From left to right: Bright-field, chlorophyll fluorescence (590–680 nm) and Venus fluoresence (520–560 nm). (d) GC-MS analysis of WT P. patens and a multi-fragment transformation resulting in the absence of the two major diterpenes found in hexane extractions, 16-ent-kaurene (1) and 16-OH-ent-kaurene (2). (e) Proper insertion and in vivo assembly of DNA fragments were confirmed by PCR. Primer pair 1 (P13 andP14) only binds in a region of DNA specific to the wild type (WT) CPS/KS locus, and gives a band of 778 bp only if the WT locus is intact. This band is absent in the three shown transformed lines, including a linearized plasmid transformant, a 50 bp overlap transformant, and a 12 bp overlap transformant. The primer pairs 2 (P14, P5), 3 (P3, P4) and 4 (P13, P2) give sizes of 1421 bp, 1882 bp, and 2372 bp respectively, indicating proper assembly of fragments and insertion in the genome. The PCR products were sequence-verified.