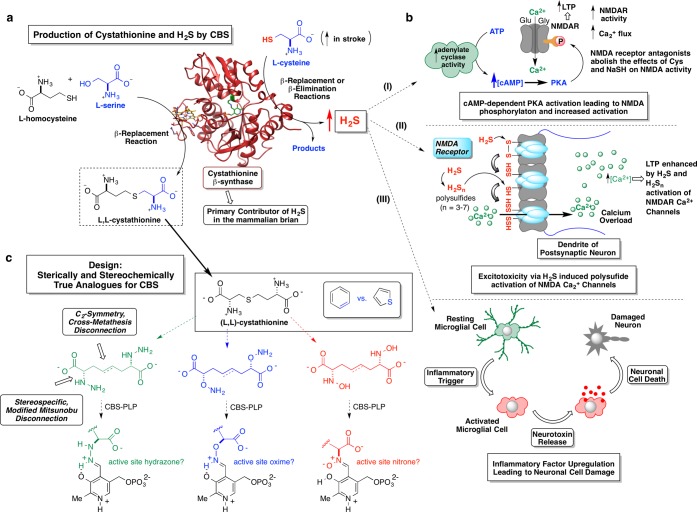
Figure 1.
(a) β-Replacement reaction mediated by PLP-dependent CBS between l-serine and l-homocysteine to produce l,l-cystathionine (lower left reaction). CBS represents the primary contributor of H2S in the mammalian brain via either β-replacement or β-elimination reactions emanating from l-cysteine (upper right reaction). (b) A few possible effects of elevated H2S levels on neurological outcomes pursuant to stroke: (i) cAMP-dependent PKA activation leading to possible NMDA receptor phosphorylation and increased activity; (ii) H2S enhancement of Ca2+ flux into the post-synaptic neuron by H2S-release from NMDA receptor-bound polysulfides (stored sulfane sulfur); (iii) following an inflammatory trigger, elevated H2S levels may augment signaling pathways involved in activating inflammatory cells (microglial cells) causing a discharge of mediators such as chemokines and cytokines that could further damage the neuron, exacerbating neuronal cell death. (c) Schematic illustrating the design principles of a library of CBS-targeted affinity-based inhibitors designed to incorporate recognition features of the CBS product, l,l-cystathionine. These inhibitor candidates were envisioned to engage the active-site PLP cofactor, forming tight binding hydrazone, oxime, or nitrone adducts.