Abstract
Purpose
Recent studies investigating the acute effects of mild traumatic brain injury (mTBI) suggest the presence of unbalanced excitatory and inhibitory mechanisms within primary motor cortex (M1). Whether these abnormalities are associated with impaired synaptic plasticity remains unknown.
Methods
The effects of continuous theta burst stimulation (cTBS) on transcranial magnetic stimulation-induced motor evoked potentials (MEPs) were assessed on average two weeks and six weeks following mTBI in five individuals.
Results
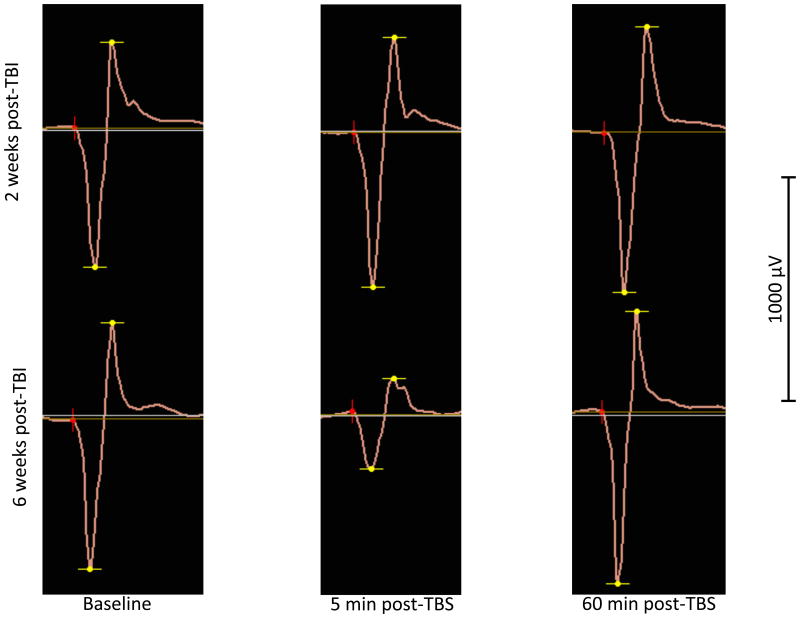
The procedure was well-tolerated by all participants. Continuous TBS failed to induce a significant reduction of MEP amplitudes two weeks after the injury, but response to cTBS normalized six weeks following injury, as a majority of patients became asymptomatic.
Conclusions
These preliminary results suggest that cTBS can be used to assess M1 synaptic plasticity in the acute and sub-acute phases following mTBI and may provide insights into neurobiological substrates of symptoms and consequences of mTBI.
Introduction
The Centers for Disease Control and Prevention estimate that between 1.4 and 3.8 millions of mild traumatic brain injuries (mTBI) occur annually in the USA (Rutland-Brown et al., 2006). Although mTBI has been long considered a short-lasting “minor” injury, current literature suggests that it may involve a clinically silent pathological process that is related to subclinical neurophysiologic and neurometabolic changes. An increasing number of studies have revealed the long term impact of mTBI or concussion since the discovery of a possible link between multiple mTBIs and the development of neurodegenerative diseases (Bazarian et al., 2009), such as Alzheimer's disease (Guskiewicz et al., 2005; McCrory, 2011; Mortimer et al., 1985; Plassman et al., 2000), chronic traumatic encephalopathy (Cantu, 2007; McCrory et al., 2007) and amyotrophic lateral sclerosis (Piazza et al., 2004). Therefore, there is a need to better understand both the acute and chronic impacts of mTBI on brain physiology to fully appreciate the timeline of the changes occurring in the brain following injury.
Insight from animal studies suggest that a complex neurometabolic cascade of events occurs in the brain in the acute phase following mTBI that involves NDMA receptors, ion channels and glutamate release (Giza & Hovda, 2001). In humans, transcranial magnetic stimulation (TMS) has been used to non-invasively assess the neurophysiological impact of mTBI as it allows precise quantification of inhibitory and excitatory systems within primary motor cortex (M1; Hallet, 2007). Using this method, previous studies have shown sometimes long-lasting disruptions in M1 inhibitory/excitatory balance, usually taking the form of increased intracortical inhibition/reduced intracortical facilitation (Chistyakov et al., 2001; De Beaumont et al., 2007, 2009; Miller et al., 2014; Pearce et al., 2014a; Powers et al. 2014; but see Tremblay et al., 2014; Pearce et al., 2014b) Moreover, chronic alterations in synaptic plasticity, possibly reflecting faulty long term potentiation (LTP) – and long term depression (LTD) - like mechanisms were found after multiple concussions, and were associated with intracortical inhibition abnormalities (De Beaumont et al., 2012). Taken together, these studies suggest the presence of impaired balance between primary motor cortex excitatory and inhibitory mechanisms following mTBI both in the acute and chronic phases, which may be related to abnormal M1 plasticity.
To our knowledge, the integrity of synaptic plasticity mechanisms following human mTBI in the acute phase has yet to be investigated. This is of major importance since impairments in M1 plasticity could prevent adaptive plastic changes to occur following injury and may themselves be the cause of pathological processes and functional disability. Continuous theta-burst stimulation (cTBS) is a repeated TMS protocol that induces long lasting reduction of corticospinal excitability and allows non-invasive and rapid assessment of motor cortex plasticity (Huang et al., 2005). Continuous TBS is thought to involve several neural mechanisms including long-term depression (LTD), and inhibitory mechanisms modulated by GABAergic transmission (Cárdenas-Morales et al., 2010). Continuous TBS can therefore provide important insight into the synaptic plasticity changes that may occur shortly after mild head trauma. The objective of the present proof-of-principle, case-series study was to provide preliminary evidence that cTBS can be safely and efficiently applied in the acute phase of mTBI to assess the integrity of synaptic plasticity mechanisms in primary motor cortex.
Methods
Participants
Case 1
This 44 year-old right-handed man was playing soccer when he sustained a head to head collision with another player and then hit the ground with his head. There was loss of consciousness (LOC) for about 90 seconds, followed by confusion, blurred vision, agitation and about 1–2 minutes of retrograde and 3–4 minutes of anterograde post-traumatic amnesia (PTA). The symptoms resolved approximately 20 minutes after the event at which point his physical and neurological exams were normal, and remained normal 10 days later. He was diagnosed with a Grade 3 concussion according to the American Academy of Neurology classification (1997). For two weeks following the accident, he complained of fatigue and poor concentration, memory problems, mild headaches and some difficulty sleeping. These symptoms had markedly improved by week 6, although he still complained of mild headaches, slight fatigue, and intermittent memory difficulties. He was not taking any drugs known to alter brain excitability, plasticity, or excitation/inhibition balance. He had a history of four prior episodes diagnosed as concussions while an athlete in college, over 20 years prior to the present episode. In two of these incidents there was no loss of consciousness, but there was varying degrees of retrograde and anterograde amnesia, mild and transient concentration and memory difficulties, headaches, and dizziness that had completely subsided within 2 months from the episode. Past medical history, review of system and family history were otherwise negative. Note that this case was previously presented in a case report by Bashir et al. (2012).
Case 2
This 24 year-old right-handed woman suffered from a bike accident during which she hit her head while wearing a helmet. She sustained a LOC of approximately 1-5 minutes duration. A brief seizure-like twitching episode (20 sec) was observed while she was unconscious. She suffered from retrograde and anterograde PTA (few minutes). A week after the injury, she was involved in a second bike accident where she hit her head again. Following this second incident, she did not report LOC, involuntary movements, seizures or retrograde/anterograde PTA. Three or four days after the second incident, she started to experience intermittent headaches and trouble concentrating. Her neurological examination was normal. She was diagnosed with a Grade 3 concussion according to the American Academy of Neurology classification (1997). She had a past medical history of attention deficit and hyperactivity disorder (ADHD) for which she was taking psychostimulant medication. She stopped taking the medication three weeks prior to the experimentation. Her past medical history, review of system and family history were otherwise negative.
Case 3
This is a 22 year-old left-handed woman who was involved in a collision with a skateboarder while she was on her bike. Following the impact, she flew over the handle bar. The front of her helmet broke and she sustained a left pre-orbital ecchymosis. The duration of the LOC is unknown. She experienced confusion and retrograde PTA for about 30 sec and anterograde amnesia for approximately 30 min. She has a past medical history of migraine. During a few days following the accident, she experienced some word finding difficulties but she did not report any increase in the frequency of her migraine or changes in her concentration. Her physical and neurological examinations were normal. She was diagnosed with a Grade 3 concussion according to the American Academy of Neurology classification (1997). She was not taking any drugs known to alter brain excitability, plasticity, or excitation/inhibition balance. Her past medical history, review of system and family history were negative.
Case 4
This is a 28 year-old right-handed woman who was involved in a car-pedestrian collision. The presence of LOC is unknown and there was no report of anterograde/retrograde PTA. Following the incident, she experienced increased intensity and frequency of headaches. Her neurological and physical exams were normal. She was diagnosed with a Grade 2 concussion according to the American Academy of Neurology classification ( 1997). A computed-tomography scan (CT-scan) of her head revealed a small right parietal subgaleal scalp hematoma along the vertex with no underlying fracture. She was not taking any drugs known to alter brain excitability, plasticity, or excitation/inhibition balance. Her past medical history, review of system and family history were negative.
Case 5
This is a 22 year-old left-handed woman who was involved in a work incident during which she was hit by a stack of plates on the left post-aural region by a co-worker. There is no report of LOC or anterograde/retrograde PTA. However, she experienced dizziness and nausea after the incident, and headaches for several days post-injury. No intracerebral anomalies were observed on the CT-scan. His neurological and physical exams were normal. He was diagnosed with a Grade 2 concussion according to the American Academy of Neurology classification (1997). She was not taking any drugs known to alter brain excitability, plasticity, or excitation/inhibition balance. His past medical history, review of system and family history were negative.
Procedure
All participants were seen within two weeks post-mTBI (M= 14 ± 3 days) and again approximately six weeks post-injury (separated by 61 ± 19 days). All participants were first seen by a neurologist and had to meet the concussion criteria of the American Academy of Neurology (Neurology, 1997). On visit 1, a brain magnetic resonance imaging (MRI) exam was performed, followed by baseline measures of TMS and the cTBS procedure. On visit 2, TMS and cTBS procedures were repeated. All participants gave their written informed consent for the study, which had been approved by the Institutional Review Board of Beth Israel Deaconess Medical Center.
TMS recordings
All participants underwent an anatomical brain MRI, using a 3-Tesla GE scanner, to rule out structural lesions and to generate high-resolution images to guide magnetic stimulation. For single-pulses, a Nexstim stimulator (Nexstim Ltd, Helsinki, Finland) was used, delivering biphasic pulses with a current flowing in the brain with an antero-posterior and then a postero-anterior (AP–PA) direction. For repetitive TMS, i.e. cTBS, a MagPro stimulator (MagVenture A/S, Farum, Denmark) was used, delivering biphasic pulses with the current flowing in an AP–PA direction. In order to ensure stable coil positioning over the stimulation site during the experimentation and to ensure that the exact same cortical location was targeted within each study session as defined by each individual's brain MRI, a Nexstim eXimia Neuronavigation system was used. During stimulation, surface electromyography (EMG) was recorded and monitored continuously on-line. Active electrodes were attached to the skin overlying the first dorsal interosseus (FDI) muscle. The reference electrode was placed over the metacarpo-phalangeal joint and a ground electrode was placed over the wrist bone or the ipsilateral forearm. EMG signals were filtered (8–500 Hz), amplified, displayed and stored off-line for analysis. The TMS system delivered triggered pulses that synchronized the TMS and EMG systems. Relaxation of the measured muscle was controlled by continuous visual EMG monitoring. Participants were asked to keep their eyes open throughout the experiment and were monitored for drowsiness.
TMS measurements
Participants were seated in a comfortable chair, with a head rest, and with their elbows flexed at approximately 90° and their hands resting on their laps. The optimal scalp location for activation of the right FDI using TMS over left primary motor cortex (M1) was determined as the location from which TMS-induced motor evoked potentials (MEPs) of maximum peak-to-peak amplitude in the right FDI. Once the optimal location was identified, a marker was placed on the MRI scan to which the individual participant was registered using the eXimia navigated brain stimulation (NBS) system. This allowed the TMS coil to be placed systematically in the same location, orientation and tilt throughout each session.
Motor threshold (MT) was determined according to the recommendations of the International Federation for Clinical Neurophysiology (Rossini et al., 1994). Single TMS pulses were delivered over the optimal scalp position at supra-threshold intensity and gradually reduced by decrements of 2% of stimulator output. Resting MT (RMT) was defined, with the Nexstim stimulator used for single-pulse TMS, as the lowest stimulus intensity capable of inducing MEPs ≥ 50 μV peak-to-peak amplitude in at least 5 of 10 consecutive trials. EMG monitoring was performed to assure that the target muscle was at rest. Prior to cTBS, active MT (AMT) was determined, and defined as the minimum single-pulse TMS intensity required to produce MEPs ≥ 200 μV in at least 5 of 10 consecutive trials while participants contracted the target muscle (contralateral FDI) at approximately 20% of maximal voluntary contraction. In order to control for prior motor contraction during the measurement of AMT, participants were asked to contract the FDI muscle approximately 2 s prior to each TMS pulse and to relax it about 1 s after each TMS pulse, for at least 3 s. The cTBS protocol was applied approximately 1 min after the end of the AMT measurement procedures; the experimenters monitored the relaxation of hand muscles continuously during and after the stimulation.
cTBS procotol
Continuous TBS was applied using parameters similar to those used by Huang et al. (Huang et al., 2005): three pulses at 50 Hz, with an interval of 200 ms between the last pulse of a triplet and the first pulse of a triplet (i.e. with an interstimulus interval of 240 ms), for a total number of 600 pulses. Thus, in the present cTBS paradigm, the triplet repetition rate was about 4.17 Hz instead of 5 Hz, both frequencies being included in the theta band. The intensity was fixed at 80% of AMT. This paradigm was recently shown to induce significant suppression of MEPs in healthy controls (see Vernet et al., 2014). Before cTBS, two to three batches of 20 to 30 MEPs (60 in total) were acquired in response to stimulation over the optimal FDI location, at an intensity of 120% of RMT and a rate of approximately 0.1 Hz (a random jitter of ±1 s was introduced to avoid any training effects). Such measures allow verifying for stability of the pre-cTBS measure of excitability; moreover, the second batch was used as the baseline to which the post-cTBS measures of excitability were compared. Following cTBS, a single batch of MEPs was measured immediately after (T0) and then at 5, 10, 20, 30, 40, 50, 60, 75 and 90 min following cTBS to track changes in amplitude over time.
Data analysis
MEP peak-to-peak amplitude was automatically determined using the Nexstim Neurophysiologic Analysis software and then visually inspected. Mean raw MEP peak-to-peak amplitudes for each time points were used for analysis. Paired-sample t-tests were conducted to assess the reproducibility of baseline MEP amplitude. A within subject repeated measure multivariate analysis of variance (MANOVA) was used to compare the impact of cTBS on MEP amplitude over time, using session (session 1 and 2) and MEP measures (11 time points) as within-group factors. Paired-sample t-tests were used to identify the effect of cTBS at the different time points in comparison to the baseline MEP measure. The critical p-value was set to 0.05. Because of the very small sample and the exploratory purpose of the present case report, no correction for multiple comparisons was applied. One participant (case 4) did not come to the second session. For statistical analyses, the missing data were replaced by the average data from the 4 other cases. All analyses were performed on raw TMS data. All statistical tests were two-tailed and performed using the Statistical Package for the Social Sciences version 21.
Results
A questionnaire was used at the beginning and at the end of each session to evaluate the presence of pain and discomfort. Two patients reported the presence of mild discomfort during the procedure. Case 2 reported, at the beginning of session 1, mild headache, for which acetaminophen was given and, at the end of session, mild neck pain. Again, at the beginning of session 2, Case 2 reported mild headache and trouble concentrating and, at the end of session 2, a mild neck pain in addition to those symptoms. Case 3 reported mild neck pain at the beginning and at the end of both sessions. Thus, the only side-effect associated with the procedure was a mild neck pain for Case 2.
A paired-sample t-test revealed no significant difference between baseline MEP measures from both session (t(4) = -.07, p = .94). The MEP response profiles in the two sessions were not parallel as indicated by a significant [session × time] interaction (F=2.23, df=10, p<0.035) (Figure 1). Subsequent paired-sample t-tests revealed no significant reduction in the MEPs size compared to baseline at all time points for session 1 (Table 1). A significant inhibition of the MEPs compared to baseline at T0, T5, T20, T50, T60, T75 and T90 was observed for session 2 (Table 2). Individual data are shown in Figure 2.
Figure 1.
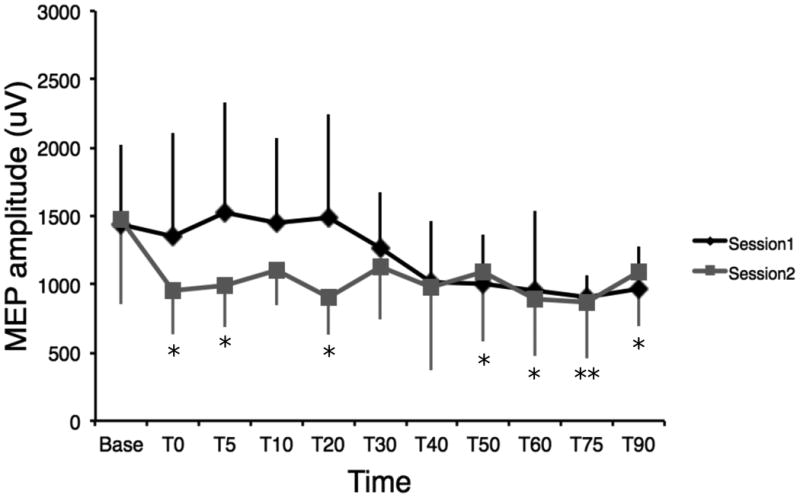
Mean MEP amplitude and standard deviations following cTBS over the different points and for session 1 and 2. Error bars show standard deviations. No significant reductions are observed on MEP amplitudes for the first session, although a small trend is observed towards the last time points. Significant reductions of MEP amplitudes are observed for sessions two for 7 out of the 10 time points. * p < 0.05; ** p < 0.01
Table 1. Paired-sample t-tests (baseline/time points) for session1.
| Time point | Mean difference (SD) | t value | p value |
|---|---|---|---|
| T0 | 86.2 (194.4) | 0.992 | 0.378 |
| T5 | −78.6 (373.2) | 0.471 | 0.662 |
| T10 | −10.8 (379.3) | 0.064 | 0.952 |
| T20 | −40.2 (244.1) | 0.368 | 0.731 |
| T30 | 173.8 (375.9) | 1.034 | 0.36 |
| T40 | 428.4 (386.9) | 2.476 | 0.069 |
| T50 | 433.6 (588.9) | 1.646 | 0.175 |
| T60 | 484.4 (573.3) | 1.889 | 0.132 |
| T75 | 536.6 (502.3) | 2.389 | 0.075 |
| T90 | 481.8 (470.8) | 2.288 | 0.084 |
Table 2. Paired-sample t-tests (baseline/time points) for session 2.
| Time point | Mean difference (SD) | t value | p value |
|---|---|---|---|
| T0 | 513.0 (302.3) | 3.940 | 0.019* |
| T5 | 477.4 (338.3) | 3.155 | 0.034* |
| T10 | 336.2 (389.8) | 2.101 | 0.104 |
| T20 | 567.6 (350.2) | 3.624 | 0.022* |
| T30 | 340.2 (290.0) | 2.623 | 0.059 |
| T40 | 494.8 (475.5) | 2.327 | 0.081 |
| T50 | 378.6 (294.3) | 2.877 | 0.045* |
| T60 | 580.4 (384.3) | 3.377 | 0.028* |
| T75 | 603.2 (280.6) | 4.807 | 0.009** |
| T90 | 383.2 (289.0) | 2.965 | 0.041* |
Note.
P<0.05;
p<0.01.
Figure 2.
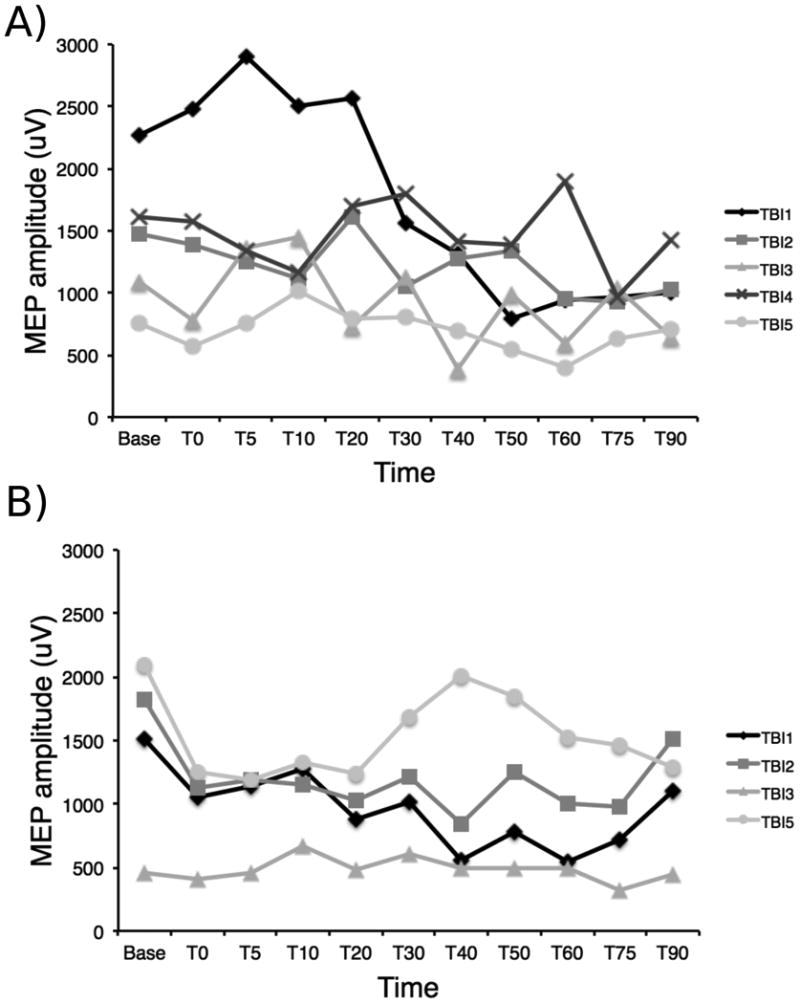
A) Individual mean MEP amplitudes for the first cTBS session. High variability is observed between the responses for each subject and therefore no clear inhibitory pattern can be visually observed. B) Individual mean MEP amplitudes for the second cTBS session. Subjects 1, 2 and 5 show a clear inhibitory response for at least the first four time points, whether only subject 3 does not seem to show an inhibitory response to the stimulation.
Discussion
The goal of this proof-of-principle study was to investigate the feasibility of using cTBS to evaluate plasticity changes in the acute and sub-acute phases of mTBI. The protocol was well-tolerated by all participants but induced a mild side-effect (neck pain) in one out of 5 patients. Preliminary results suggest the presence of altered plasticity 2 weeks post-mTBI, as cTBS failed to elicit the usual suppression of MEPs post-stimulation, which could reflect altered M1 LTD-like mechanisms. Significant cTBS-related suppression of MEPs was observed 6 weeks post-mTBI suggesting a resolution of plasticity abnormalities beyond the acute phase.
A common observation following mTBI is the presence of altered M1 intracortical excitability in the acute (Chistyakov et al., 2001; Pearce et al., 2014b; Miller et al., 2014; Powers et al., 2014) and chronic (De Beaumont et al., 2007,2009; Tremblay et al., 2011; Pearce et al., 2014b) phases of injury. More specifically, increased intracortical inhibition (Chistyakov et al., 2001; Pearce et al., 2014b; Miller et al., 2014) and decreased intracortical facilitation (Powers et al., 2014) have been reported in the acute and sub-acute phases of mTBI. Despite strong evidence suggesting inhibitory/excitatory imbalance in the primary motor cortex of individuals with mTBI, the duration of such effects is unclear. Pearce and collaborators (2014b) found increased GABA-related inhibition 48h and 96h after concussion that normalized 10 days post-injury, whereas Miller et al. (2014) reported similarly increased inhibition that lasted up to 2 months after the concussive event. Intracortical inhibition has also been reported to be increased 1-4 weeks (Powers et al., 2014) and 9 months after a concussion (De Beaumont et al., 2007) and within normal values 41 months post-injury (Tremblay et al., 2014).
In the present study, we show reduced synaptic plasticity in the acute phase as indexed by the response to cTBS, and that this this abnormality disappears six weeks post-injury. An association between abnormal intracortical excitability and aberrant synaptic plasticity has been previously shown in concussed athletes on average 14 months post-injury. De Beaumont et al. (2012) reported that increased silent period durations in concussed athletes, presumably reflecting faulty GABAB transmission, were negatively correlated with the level of synaptic plasticity induced with paired associative stimulation. In the present study, the hypoexcitatory or hyperinhibitory state of M1 intracortical networks could prevent the injured brain from responding adequately to the effects of cTBS and therefore be an accurate marker of early abnormal plasticity. The inability of the injured brain to respond to cTBS appears short-lived, however, which is in contradiction with the previous study by De Beaumont and collaborators (2012) who showed persistent motor cortex LTD- and LTP-like deficits in the chronic phase following sport concussion. This discrepancy could be explained by the fact that the current sample included 4 individuals with mTBI who did not have a history of multiple concussions (3 and over) and that were not subjected to recurrent sub-concussive blows through contact sports. More studies are needed to determine if sport-related mTBIs involve a specific pattern of brain response linked to repeated concussive and sub-concussive hits to the head.
Animal studies have shown that bursts of 3-5 pulses at 50-100 Hz (theta rhythm) induce LTP/LTD when applied to the motor cortex or hippocampus (Hess & Donoghue, 1996; Larson, et al., 1986). While the exact mechanism underlying the effects of cTBS on the human brain are still unknown, it has been suggested that MEP suppression following stimulation could be related to long-term depression (LTD)-like processes mediated by N-methyl-D-aspartate receptors (NMDA-r), as NMDA-r antogatonist memantine was shown to block the after effects of cTBS (Huang et al., 2007). Modulation of GABA receptors (Thickbroom, 2007) and glutamate receptors (Glu-r) (Huang et al., 2007) has also been proposed as a possible mechanism explaining excitability changes following TBS. TBS could therefore target both excitatory and inhibitory networks within the human motor cortex (Cárdenas-Morales et al., 2010). The present data are in line with this hypothesis since M1 alterations in glutamate (Babikian et al., 2006; Henry et al, 2010; Shutter et al., 2004) and abnormal interactions between M1 GABA and glutamate (Tremblay et al., 2014) have been shown in the acute and chronic phases of TBI and sport-related mTBI using magnetic resonance spectroscopy. Abnormal GABA and glutamate transmission could therefore partly explain the inhibitory/excitatory imbalance found in the motor cortex of individuals with mTBI and its associated effects on synaptic plasticity.
Continuous TBS has been used with various populations to non-invasively probe synaptic plasticity in the conscious human brain. This method has many advantages over other techniques such as its short application time (Huang et al., 2005), low intensity of stimulation (Huang et al., 2005) and reasonable intra-subject reproducibility over two seperate sessions (Vernet et al., 2014). As recent studies have suggested that altered metabolite interactions and plasticity mechanisms within M1 could be a key feature of mTBI pathophysiology, the goal of the present proof-of-principle study was to determine whether cTBS could be used to assess the integrity of plasticity mechanisms in the acute and sub-acute phases of mTBI. Reduced LTD-like synaptic plasticity was found two weeks following injury and disappeared six weeks post-injury. Whether the altered LTD-like plasticity mechanisms seen in the acute phase following mTBI is part of the pathophysiology of the injury or reflects a compensatory mechanism of short-duration needs to be assessed in larger prospective studies and compared to normative values.
Acknowledgments
We thank Frederick Ifert-Miller, Anna-Katherine Brem, and Woo-Kyoung Wu for their contribution. This study was supported in part by grants from the Center for Integration of Medicine and Innovative Technology (CIMIT), the Canadian Institutes of Health Research and the Fonds de Recherche du Québec – Santé, Grant Number 8 UL1 TR000170, Harvard Clinical and Translational Science Center, from the National Center for Advancing Translational Science, and the Football Players Health Study at Harvard University. ST was supported by a Vanier Canada Graduate Scholarship from the Canadian Institutes of Health Research. MV was supported by the Fyssen Foundation. APL was supported in part by grants from the Sidney R. Baer Jr. Foundation, the National Institutes of Health (R01HD069776, R01NS073601, R21 MH099196, R21 NS082870, R21 NS085491, R21 HD07616), and Harvard Catalyst | The Harvard Clinical and Translational Science Center (NCRR and the NCATS NIH , UL1 RR025758). The content is solely the responsibility of the authors and does not necessarily represent the official views of Harvard Catalyst, Harvard University and its affiliated academic health care centers, the Canadian Institutes of Health, the National Institutes of Health or the Sidney R. Baer Jr. Foundation.
Footnotes
Conflict of interest statement: Dr. Pascual- Leone serves on the scientific advisory boards for Nexstim, Neuronix, Starlab, Neuroelectrics, Axilum Robotics, Magstim, and Neosync; and is listed as an inventor on several issued and pending patents on the real-time integration of transcranial magnetic stimulation (TMS) with electroencephalography (EEG) and magnetic resonance imaging (MRI).
References
- Babikian T, Freier MC, Ashwal S, Riggs ML, Burley T, Holshouser BA. MR spectroscopy: predicting long-term neuropsychological outcome following pediatric TBI. J Magn Res Imaging. 2006;24(4):801–811. doi: 10.1002/jmri.20696. [DOI] [PubMed] [Google Scholar]
- Bashir S, Vernet M, Yoo WK, Mizrahi I, Théoret H, Pascual-Leone A. Changes in cortical plasticity after mild traumatic brain injury. Restor Neurol Neurosci. 2012;30(4):277–282. doi: 10.3233/RNN-2012-110207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bazarian JJ, Cernak I, Noble-Haeusslein L, Potolicchio S, Temkin N. Long-term neurologic outcomes after traumatic brain injury. J Head Trauma Rehab. 2009;24(6):439–451. doi: 10.1097/HTR.0b013e3181c15600. [DOI] [PubMed] [Google Scholar]
- Cantu RC. Chronic traumatic encephalopathy in the National Football League. Neurosurg. 2007;61(2):223–225. doi: 10.1227/01.NEU.0000255514.73967.90. [DOI] [PubMed] [Google Scholar]
- Cárdenas-Morales L, Nowak DA, Kammer T, Wolf RC, Schönfeldt-Lecuona C. Mechanisms and applications of theta-burst rTMS on the human motor cortex. Brain Topogr. 2010;22(4):294–306. doi: 10.1007/s10548-009-0084-7. [DOI] [PubMed] [Google Scholar]
- Creeley CE, Wozniak DF, Bayly PV, Olney JW, Lewis LM. Multiple episodes of mild traumatic brain injury result in impaired cognitive performance in mice. Acad Emerg Med. 2004;11(8):809–819. doi: 10.1111/j.1553-2712.2004.tb00761.x. [DOI] [PubMed] [Google Scholar]
- De Beaumont L, Lassonde M, Leclerc S, Théoret H. Long-term and cumulative effects of sports concussion on motor cortex inhibition. Neurosurg. 2007;61(2):329–37. doi: 10.1227/01.NEU.0000280000.03578.B6. [DOI] [PubMed] [Google Scholar]
- De Beaumont L, Théoret H, Mongeon D, Messier J, Leclerc S, Tremblay S, et al. Brain function decline in healthy retired athletes who sustained their last sports concussion in early adulthood. Brain. 2009;132(Pt 3):695–708. doi: 10.1093/brain/awn347. [DOI] [PubMed] [Google Scholar]
- De Beaumont L, Tremblay S, Poirier J, Lassonde M, Théoret H. Altered Bidirectional Plasticity and Reduced Implicit Motor Learning in Concussed Athletes. Cereb Cortex. 2012;22:112–121. doi: 10.1093/cercor/bhr096. [DOI] [PubMed] [Google Scholar]
- DeFord SM, Wilson MS, Rice AC, Clausen T, Rice LK, Barabnova A, et al. Repeated mild brain injuries result in cognitive impairment in B6C3F1 mice. J Neurotrauma. 2002;19(4):427–438. doi: 10.1089/08977150252932389. [DOI] [PubMed] [Google Scholar]
- Eggers C, Fink GR, Nowak DA. Theta burst stimulation over the primary motor cortex does not induce cortical plasticity in Parkinson's disease. J Neurol. 2010;257(10):1669–1674. doi: 10.1007/s00415-010-5597-1. [DOI] [PubMed] [Google Scholar]
- Giza CC, Hovda DA. The Neurometabolic Cascade of Concussion. J Athl Train. 2001;36(3):228–235. [PMC free article] [PubMed] [Google Scholar]
- Guskiewicz KM, Marshall SW, Bailes J, McCrea M, Cantu RC, Randolph C, Jordan BD. Association between recurrent concussion and late-life cognitive impairment in retired professional football players. Neurosurg. 2005;57(4):719–26. doi: 10.1093/neurosurgery/57.4.719. [DOI] [PubMed] [Google Scholar]
- Hallet M. Transcranial magnetic stimulation: a primer. Neuron. 2007;55:187–99. doi: 10.1016/j.neuron.2007.06.026. [DOI] [PubMed] [Google Scholar]
- Henry LC, Tremblay S, Boulanger Y, Ellemberg D, Lassonde M. Neurometabolic changes in the acute phase after sports concussions correlate with symptom severity. J Neurotrauma. 2010;27(1):65–76. doi: 10.1089/neu.2009.0962. [DOI] [PubMed] [Google Scholar]
- Hess G, Donoghue JP. Long-term potentiation and long-term depression of horizontal connections in rat motor cortex. Acta Neurobiol Exp. 1996;56(1):397–405. doi: 10.55782/ane-1996-1143. [DOI] [PubMed] [Google Scholar]
- Huang YZ, Chen RS, Rothwell JC, Wen HY. The after-effect of human theta burst stimulation is NMDA receptor dependent. Clin Neurophysiol. 2007;118(5):1028–1032. doi: 10.1016/j.clinph.2007.01.021. [DOI] [PubMed] [Google Scholar]
- Huang YZ, Edwards MJ, Rounis E, Bhatia KP, Rothwell JC. Theta burst stimulation of the human motor cortex. Neuron. 2005;45(2):201–206. doi: 10.1016/j.neuron.2004.12.033. [DOI] [PubMed] [Google Scholar]
- Larson J, Wong D, Lynch G. Patterned stimulation at the theta frequency is optimal for the induction of hippocampal long-term potentiation. Brain Res. 1986;368(2):347–350. doi: 10.1016/0006-8993(86)90579-2. [DOI] [PubMed] [Google Scholar]
- McCrory P. Sports concussion and the risk of chronic neurological impairment. Clin J Sport Med. 2011;21(1):6–12. doi: 10.1097/JSM.0b013e318204db50. [DOI] [PubMed] [Google Scholar]
- McCrory P, Zazryn T, Cameron P. The evidence for chronic traumatic encephalopathy in boxing. Sports Med. 2007;37(6):467–476. doi: 10.2165/00007256-200737060-00001. [DOI] [PubMed] [Google Scholar]
- Mortimer JA, French LR, Hutton JT, Schuman LM. Head injury as a risk factor for Alzheimer's disease. Neurology. 1985;35(2):264–267. doi: 10.1212/wnl.35.2.264. [DOI] [PubMed] [Google Scholar]
- Neurology AAO. Practice parameter: the management of concussion in sports (summary statement). Report of the Quality Standards Subcommittee. Neurology. 1997;48(3):581–585. doi: 10.1212/wnl.48.3.581. [DOI] [PubMed] [Google Scholar]
- Pearce AJ, Hoy K, Rogers MA, Corp DT, Maller JJ, Drury HGK, et al. The Long-Term Effects of Sports Concussion on Retired Australian Football Players: A Study Using Transcranial Magnetic Stimulation. J Neurotrauma. 2014a;31(13):1139–45. doi: 10.1089/neu.2013.3219. [DOI] [PubMed] [Google Scholar]
- Pearce AJ, Hoy K, Rogers MA, Corp DT, Davies CB, Maller JJ, et al. Acute motor, neurocognitive and neurophysiological change following injury in Australian amateur football. A prospective multimodal investigation. J Sci Med Sport. 2014b doi: 10.1016/j.jsams.2014.07.010. [DOI] [PubMed] [Google Scholar]
- Piazza O, Sirén AL, Ehrenreich H. Soccer, neurotrauma and amyotrophic lateral sclerosis: is there a connection? Curr Med Res Opin. 2004;20(4):505–508. doi: 10.1185/030079904125003296. [DOI] [PubMed] [Google Scholar]
- Plassman BL, Havlik RJ, Steffens DC, Helms MJ, Newman TN, Drosdick D, et al. Documented head injury in early adulthood and risk of Alzheimer's disease and other dementias. Neurology. 2000;55(8):1158–1166. doi: 10.1212/wnl.55.8.1158. [DOI] [PubMed] [Google Scholar]
- Powers KC, Cinelli ME, Kalmar JM. Cortical hypoexcitability persists beyond the symptomatic phase of a concussion. Brain Injury. 2014;28(4):465–471. doi: 10.3109/02699052.2014.888759. [DOI] [PubMed] [Google Scholar]
- Rioult-Pedotti MS, Friedman D, Donoghue JP. Learning-induced LTP in neocortex. Science. 2000;290(5491):533–536. doi: 10.1126/science.290.5491.533. [DOI] [PubMed] [Google Scholar]
- Rossini PM, Barker AT, Berardelli A, Caramia MD, Caruso G, Cracco RQ, et al. Non-invasive electrical and magnetic stimulation of the brain, spinal cord and roots: basic principles and procedures for routine clinical application. Report of an IFCN committee. Electroencephal Clin Neurophysiol. 1994;91(2):79–92. doi: 10.1016/0013-4694(94)90029-9. [DOI] [PubMed] [Google Scholar]
- Rutland-Brown W, Langlois JA, Thomas KE, Xi YL. Incidence of traumatic brain injury in the United States, 2003. J Head Trauma Rehab. 2006;21(6):544–548. doi: 10.1097/00001199-200611000-00009. [DOI] [PubMed] [Google Scholar]
- Schwarzbach E, Bonislawski DP, Xiong G, Cohen AS. Mechanisms underlying the inability to induce area CA1 LTP in the mouse after traumatic brain injury. Hippocampus. 2006;16(6):541–550. doi: 10.1002/hipo.20183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shutter L, Tong KA, Holshouser BA. Proton MRS in acute traumatic brain injury: role for glutamate/glutamine and choline for outcome prediction. J Neurotrauma. 2004;21(12):1693–1705. doi: 10.1089/neu.2004.21.1693. [DOI] [PubMed] [Google Scholar]
- Suppa A, Marsili L, Belvisi D, Conte A, Iezzi E, Modugno N, et al. Lack of LTP-like plasticity in primary motor cortex in Parkinson's disease. Exp Neurol. 2011;227(2):296–301. doi: 10.1016/j.expneurol.2010.11.020. [DOI] [PubMed] [Google Scholar]
- Thickbroom GW. Transcranial magnetic stimulation and synaptic plasticity: experimental framework and human models. Exp Brain Res. 2007;180(4):583–593. doi: 10.1007/s00221-007-0991-3. [DOI] [PubMed] [Google Scholar]
- Tremblay S, Beaulé V, Proulx S, Tremblay S, Marjańska M, Doyon J, et al. Multimodal assessment of primary motor cortex integrity following sport concussion in asymptomatic athletes. Clin Neurophysiol. 2014;125(7):1371–1379. doi: 10.1016/j.clinph.2013.11.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremblay S, De Beaumont L, Lassonde M, Théoret H. Evidence for the Specificity of Intracortical Inhibitory Dysfunction in Asymptomatic Concussed Athletes. J Neurotrauma. 2011;28:493–502. doi: 10.1089/neu.2010.1615. [DOI] [PubMed] [Google Scholar]
- Vernet M, Bashir S, Yoo WK, Oberman L, Mizrahi I, Ifert-Miller F, et al. Reproducibility of the effects of theta burst stimulation on motor cortical plasticity in healthy participants. Clin Neurophysiol. 2014;125(2):320–326. doi: 10.1016/j.clinph.2013.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]