Table 5.
Optimization of B Ring substituents
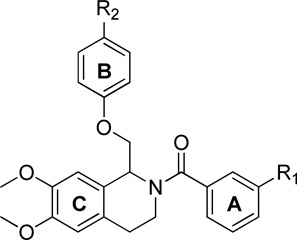 | ||||||
|---|---|---|---|---|---|---|
| I10 µM / ICONTROL (mean ± SEM, %) |
EC50 (maximal potentiation) (µM, %)a |
|||||
| R1 | R2 | GluN2C | GluN2D | GluN2C | GluN2D | |
| 1 | H | OMe | 116 ± 2.9 | 123 ± 2.3 | 12 (145%) | 11 (156%) |
| 2 | Cl | OMe | 193 ± 7.3 | 179 ± 5.7 | 4.6 (233%) | 5.0 (215%) |
| 100 | Cl | OEt | 93 ± 5.5 | 92 ± 1.4 | -- | -- |
| 101 | Br | OEt | 99 ± 2.0 | 81 ± 1.7 | -- | -- |
| 102 | Cl | OH | 105 ± 1.9 | 98 ± 0.8 | -- | -- |
| 103 | H | SMe | 102 ± 5.3 | 100 ± 1.9 | -- | -- |
| 104 | Cl | SMe | 164 ± 6.7 | 143 ± 3.9 | 5.1 (191%) | 5.3 (159%) |
| 205 | H | Et | 120 ± 2.2 | 108 ± 2.6 | 23 (160%) | -- |
| 105 | Cl | OCF3 | 102 ± 1.1 | 97 ± 1.5 | -- | -- |
| 131 | H | NH2 | 102 ± 4.4 | 93 ± 0.9 | -- | -- |
| 132 | Cl | NH2 | 97 ± 1.8 | 100 ± 1.1 | -- | -- |
| 133 | Br | NH2 | 93 ± 0.8 | 95 ± 0.7 | -- | -- |
| 134 | Cl | NHMe | 97 ± 0.9 | 97 ± 1.7 | -- | -- |
| 135 | Br | NHMe | 108 ± 2.3 | 100 ± 1.1 | -- | -- |
| 106 | H | NMe2 | 99 ± 2.0 | 97 ± 2.0 | -- | -- |
| 107 | Cl | NMe2 | 120 ± 3.4 | 110 ± 1.3 | 35 (198%) | -- |
| 108 | Br | NMe2 | 127 ± 4.1 | 108 ± 1.5 | 12 (167%) | -- |
| 206 | Cl | CO2Me | 103 ± 2.3 | 107 ± 1.7 | -- | -- |
| 207 | Br | CO2Me | 107 ± 4.0 | 102 ± 0.8 | -- | -- |
| 109 | Cl | OBn | 93 ± 1.6 | 93 ± 2.5 | -- | -- |
| 128 | H | NO2 | 94 ± 2.0 | 91 ± 1.7 | -- | -- |
| 129 | Cl | NO2 | 90 ± 0.7 | 93 ± 0.8 | -- | -- |
| 130 | Br | NO2 | 95 ± 3.4 | 92 ± 2.6 | -- | -- |
| 208 | F | F | 85 ± 1.3 | 86 ± 1.2 | -- | -- |
Fitted EC50 values are shown to two significant digits when potentiation at 10 µM test compound exceeded 115%; values in parentheses are the fitted maximum response as a percentage of the initial glutamate (100 µM) and glycine (30 µM) response. Data are from between 5–25 oocytes from 2–5 frogs for each compound. Compounds 1 and 2 were also shown in Tables 1–4, and are included here for comparison.
