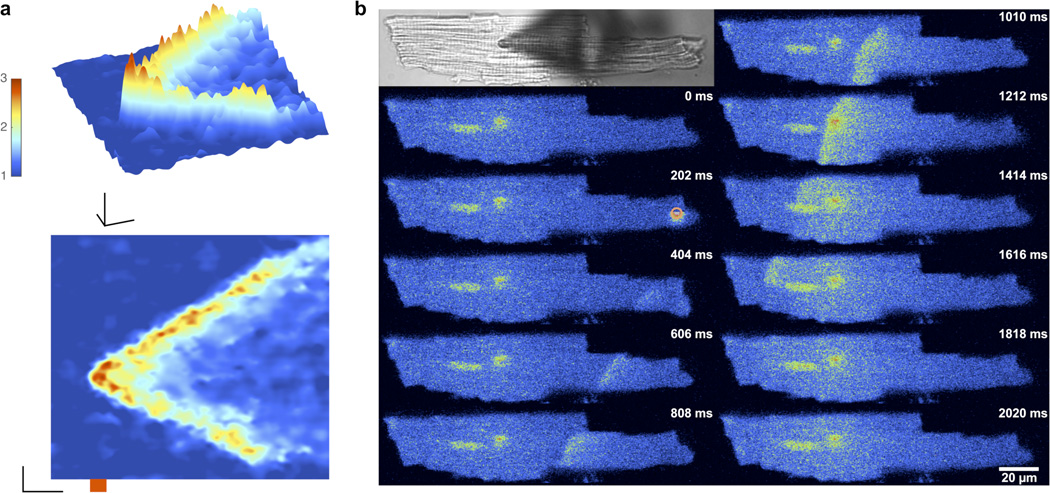
Figure 4. Visible light and 2P excitation of BIST-2EGTA:Ca2+ complex in cardiac myocytes initiates intracellular Ca2+ waves.
Single cardiac myocytes were loaded via a patch pipette with BIST-2EGTA and X-rhod-5F or rhod-2. Changes in [Ca2+]free were monitored in line scan (x,t) mode (2.116 ms per line) using laser-scanning confocal microscopy at 561 nm after 2P uncaging at the center of the line or by whole cell frame scan (202 ms per frame, 406 × 96 pixels) imaging after uncaging with visible light. A mode-locked Ti:sapphire laser tuned to 810 nm was used for 2P excitation (20 mW, 20 ms). A 405-nm continuous-wave laser was used for uncaging with visible light (10 mW, 100 ms). Line scan data are displayed as 3D surface plots (time in x, space in y and fluorescence as F/F0 on a pseudo-color scale in z) with the corresponding 2D plots (time in x and distance in y) below, (a) Rapid line scan confocal imaging revealed 2P excitation (20 ms, red bar) could initiate Ca2+ signals that propagated extensively in both directions away from the initial uncaging position. Scale bars for units of 1 F/F0, 5 µn and 50 ms. (b) Uncaging with visible light (orange circle) initiated a Ca2+ wave that propagated throughout the cell. Top left is a transmitted light image of the cell with the pipette seen as a shadow. Pseudocolor images represent raw fluorescence intensity data. Frame sequence is top left to bottom left, followed by top right to bottom right. The nucleus and patch pipette can be seen as bright structures in the left portion of the cell. The time stamp is from the beginning of each frame.