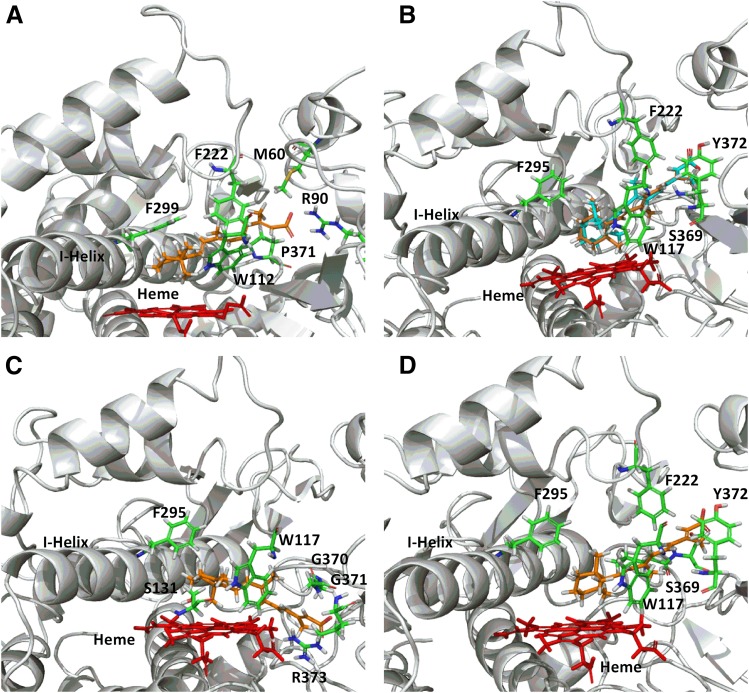
Fig. 3.
CYP26A1 (A) and CYP26B1 (B–D) homology models with at-RA docked in the active site. A single docking orientation of at-RA in the CYP26A1 homology model accounted for 4-, 16-, and 18-hydroxylation of at-RA and suggested that abstraction of the hydrogen atom leading to formation of 4-(S)-hydroxy-at-RA was the preferred binding orientation for CYP26A1, supporting the reported stereoselective metabolism of at-RA by CYP26A1 (A). Alternatively, docking of at-RA in the active site of CYP26B1 suggested that orientation of either hydrogen atom at the prochiral 4-position of the beta-ionone ring toward the heme iron was equally favorable, in agreement with the observed formation of both 4-(R)-hydroxy- (orange structure) and 4-(S)-hydroxy-at-RA (cyan structure) by CYP26B1 (B). The CYP26B1 model was also capable of docking at-RA such that the 16-hydroxy- or 18-hydroxyretinoic acid metabolites would be the predicted metabolite products (C and D).